Solutions in Vascular Surgery
Advanced Placental-Based Allografts When Patients Are in Need
There are many risk-factors to healing such as patient co-morbidities and complex defects.1 MIMEDX offers an array of advanced placental-based allografts that may be used in a variety of surgical applications to provide valuable benefits in patient care.
Video Testimonials
Clinical Use Examples
- Complex soft-tissue defects
- Debridement/dehiscence
- Complex incision management
- Amputations
- Bridge to STSG
- STSG
- Exposed bone and tendon
Product Advantages: AMNIOEFFECT, AMNIOFIX, and AMNIOCORD
-
Provide a protective barrier and environment that supports the healing cascade
-
Protect the wound bed to aid in the development of granulation tissue
-
Provide a human biocompatible extracellular matrix (ECM) and contain 250+ regulatory proteins2-5
-
Shelf-stable* with a 5-year shelf life
-
Compatible with negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy (HBOT)
*See Instructions for Use
AXIOFILL:
- A lyophilized, biocompatible, and human extracellular matrix derived from the placental disc.
- For use in the replacement or supplementation of damaged or inadequate integumental tissue.
- Versatile and easy to use: apply dry or hydrated as a paste.
- Early scientific data in a nude mouse model shows AXIOFILL supports site appropriate function in tissue by allowing cell attachment, new collagen formation on the matrix, and new blood vessel formation.6
Case Studies:
How Physicians Use Our Products
Case study 1
Left Lower Extremity (LLE) with AMNIOFIX, EPIFIX, and Negative Pressure Wound Therapy (NPWT)7
Challenge
Challenges in critical limb ischemia extend beyond those limited to the extremity itself. Patients with atherosclerotic risk factors tend to have multi-vessel disease, such as coronary artery disease (CAD) and peripheral artery disease (PAD). Patients with complex wounds, who would otherwise be ideal candidates for lower extremity bypass, often cannot undergo general anesthesia and thus are left with endovascular therapy and advanced wound therapies as their remaining options for limb salvage as an alternative to undergoing primary amputation.
Clinical History
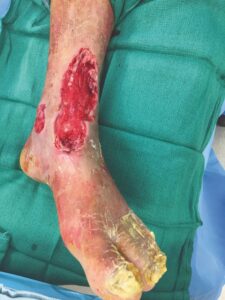
A 62-year-old male presented to the ER with chest pain and chronic left lower extremity wounds that extended from the lower leg onto the forefoot and on the medial malleolus (Figure 1). The patient also had a history of myocardial infarction, hypertension, diabetes, smoking, severe PAD and prior failed endovascular therapy at another institution. The patient was evaluated for chest pain and was found to have severe CAD. The cardiothoracic surgeons would not intervene surgically because of his underlying LLE necrosis and infection. Vascular surgery was consulted for a lower extremity bypass, but the patient was not selected as a surgical candidate for general anesthesia due to his severe CAD and other comorbidities. The patient was therefore taken for repeat endovascular therapy including atherectomy of the left superficial femoral artery (SFA), angioplasty, and placement of two SFA stents, which successfully revascularized flow to the foot.
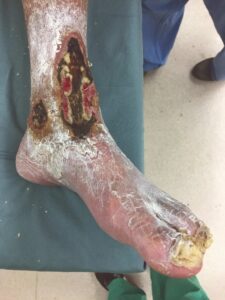
Figure 1: LLE wounds at presentation
Surgical Intervention
The patient was taken to the OR for wound debridement two days later. The necrosis involved the subcutaneous tissues and portions of the extensor digitorum longus tendon, but there was no bone exposure. Due to the patient’s high risk status and impaired closure status, AMNIOFIX was applied in conjunction with NPWT.
AMNIOFIX is a dehydrated human amnion/chorion membrane allograft. AMNIOFIX sheets provide a protective barrier that supports the healing cascade. It also protects the wound bed to aid in the development of granulation tissue in chronic and acute wounds. AMNIOFIX provides a human biocompatible extracellular matrix (ECM) and contains 300+ regulatory proteins.2-4
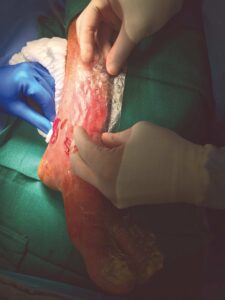
A 6 cm x 16 cm AMNIOFIX graft was perforated for exudate pass through and cut to fit both the large area wound on the forefoot and the ankle wound. The grafts were covered with a non-adherent gauze dressing. A negative pressure foam dressing was then cut to size, applied over the non-adherent gauze, sealed, and set to the recommended pressure settings (Figures 2-4). Dressings were left in place for five days before the first NPWT dressing change. At day nine, the non-adherent dressing was removed and good early granulation tissue formation was observed. A future skin graft procedure was being considered for wound closure, but this option was ultimately avoided as the wound improved over the following weeks.
 |
 |
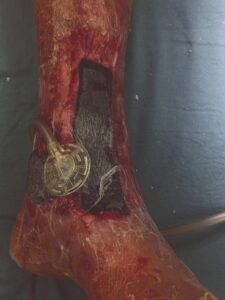 |
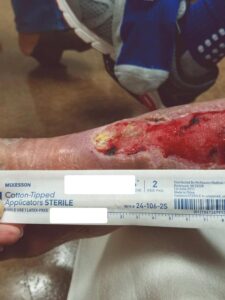 Figure 5: Week 2 s/p 1st EPIFIX treatment Figure 5: Week 2 s/p 1st EPIFIX treatment |
Figure 2–4: AMNIOFIX used in conjunction with NPWT
Follow-Up
At the two week follow-up, healthy granulation tissue was observed throughout (Figure 5). EPIFIX was applied in the office and the allografts were again left in place for five days before normal NPWT dressing changes resumed (Figure 6).
EPIFIX is a dehydrated human amnion/chorion membrane allograft. EPIFIX sheets provide a protective barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic wounds. EPIFIX provides a biocompatible human extracellular matrix and contains 300+ regulatory proteins.2-4
Seven weeks after the initial endovascular therapy, surveillance duplex scanning showed recurrent anterior tibial stenosis, which was then successfully treated with atherectomy and angioplasty to optimize perfusion. A final EPIFIX application was done on week nine and the wound progression to closure continued with the exception of a dime sized area on the LLE with exposed tendon seen on Week 13 (Figure 7). At that point, NPWT was discontinued and the remaining area was treated with conventional dressings and was fully closed and stable at follow-up one month later (Figure 8).
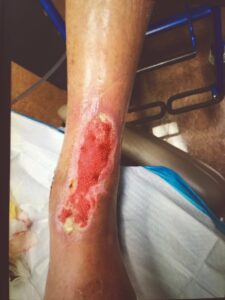 Figure 6: Week 4 s/p 2nd EPIFIX treatment |
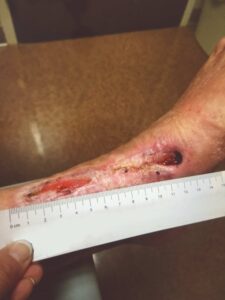 Figure 7: Week 13 s/p 3rd EPIFIX treatment |
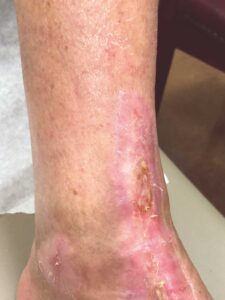 Figure 8: Week 17 wound completely closed |
Conclusion
This case demonstrates an appropriate use of advanced therapies to gain additional treatment options for a very high-risk patient that was not a surgical candidate, but would have otherwise benefited from a bypass procedure. AMNIOFIX and EPIFIX, in conjunction with successful endovascular therapy and NPWT were used. The patient ultimately avoided a likely below the knee amputation or skin grafting procedure to close the wounds. Overall, the time to wound closure in this patient was impressive given the extensive nature of his wounds.
NOTE- No studies have been conducted to determine optimal MIMEDX allograft usage in conjunction with NPWT. Description based on author’s personal experience with both products.
Case study 2
BKA After Prior Amputation Dehiscence with AMNIOFIX7
Challenge
Despite advances in vascular surgery, endovascular therapy, and soft-tissue management, closure of diseased limbs remains challenging. The primary goal of limb salvage is to restore and maintain ambulation. However, closure challenges can further complicate the clinician’s efforts. Challenges to wound closure in this setting include non-compliance, PAD, hygiene, smoking, multiple comorbidities, etc. Reported closure rates for transmetatarsal amputations (TMA) range from 40-70%,8 while reoperation rates range from 8 to 63%, with approximately one-third resulting in a major amputation.9 Once other more conservative limb salvage options have been exhausted, BKA can still offer the patient a relatively functional limb for use with prosthetic to ambulate.
Clinical History
The patient is a 56-year-old female with a history of severe peripheral artery disease, uncontrolled diabetes, end stage renal disease on dialysis, CAD with a prior percutaneous coronary intervention, and diabetic foot ulcers that led to a TMA by podiatry. She did not have the appropriate arterial revascularization at the time and the TMA site dehisced due to pressure from walking and the impaired closure from severe comorbidities. The wound worsened with necrosis and infection, to the point where re-approximation of the tissue was no longer possible (Figure 1).
The patient was referred to vascular surgery for her lower limb PAD. Atherectomy and angioplasty were performed to address her superficial femoral artery stenosis and anterior tibial artery stenosis, which successfully improved perfusion to the foot with three vessel runoff. The wound would still not heal after one month with multiple debridements and treatments by podiatry. The patient was referred back to vascular surgery and scheduled for a BKA.
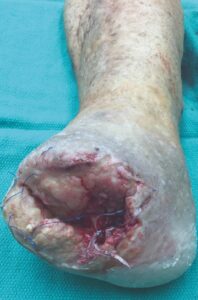 |
Figure 1: Non-healing dehiscence after TMA
Surgical Intervention
A standard BKA was performed. Based on the patient’s history of an inability to close wounds in the setting of adequate perfusion, AMNIOFIX was used.
AMNIOFIX is a dehydrated human amnion/chorion membrane allograft. AMNIOFIX sheets provide a protective barrier that supports the healing cascade. It also protects the wound bed to aid in the development of granulation tissue in chronic and acute wounds. AMNIOFIX provides a human biocompatible extracellular matrix and contains 300+ regulatory proteins.2-4
A 6 cm x 16 cm AMNIOFIX allograft was cut into several pieces and placed into the surgical site. One portion of the allograft was placed on the muscular bed, where the AMNIOFIX allograft would be positioned between muscle and bone after the subcutaneous tissue was closed (Figure 2). The other portions were placed in the subcutaneous space before dermal closure in this high risk suture line (Figures 3-5).
|
Figure 2: AMNIOFIX placed on muscular bed |
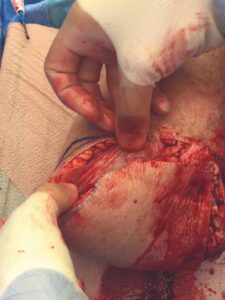 Figure 3: AMNIOFIX in subcutaneous tissue before closure |
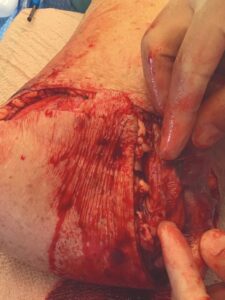 Figure 4: AMNIOFIX in subcutaneous tissue before closure |
Figure 5: Flap closure |
Follow-Up
The patient fell onto her stump in the hospital five days postoperatively. Despite the fall and resultant mild dehiscence, there was no swelling and the stump remained intact (Figure 6). This type of injury could have resulted in amputation failure. After thirty days, the BKA incision was fully closed. The patient was again seen eleven weeks post op and the amputation remained stable (Figure 7).
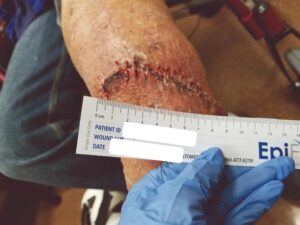 Figure 6: s/p fall onto stump on Day 5 |
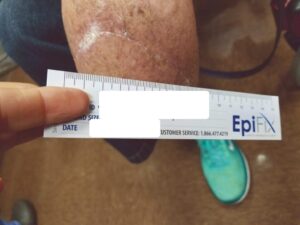 Figure 7: Wound fully closed |
Conclusion
Case study 3
Lower Extremity Bypass Dehiscence with EPIFIX and NPWT7
Challenge
Data suggests that soft-tissue complications following lower extremity bypass surgery are observed in >11% of cases. Notably, soft-tissue complications are one of the most common reasons for hospital readmission within 30 days.10 Dehiscence in lower extremity bypass patients commonly occurs secondary to an underlying seroma, hematoma, or tissue flap created during saphenous vein harvest. These defects can be challenging to close as they often extend along the entire limb. Furthermore, closing these defects in an expedited fashion helps preserve graft integrity and patency, thus avoiding potential graft infection and or occlusion.
Clinical History
A 78-year-old male presented with a right first toe necrosis and underwent endovascular therapy for known superficial femoral artery (SFA) occlusion. The patient had multiple comorbidities including diabetes, hypertension, hyperlipidemia, PAD, and prior smoking. The initial revascularization was successful, but upon follow-up, duplex surveillance showed recurrent occlusion. He subsequently underwent a right femoral to above the knee popliteal bypass with a greater saphenous vein graft. Intraoperatively, a drain was placed along the harvest site. The drain was removed on postop day four. At that time, the incision was intact with no fluid collection observed. Unlike our current practice when treating high-risk patients, no AMNIOFIX allografts were placed in the surgical site at the time of the bypass procedure.
During his postoperative hospitalization, his venous bypass was found to be occluded secondary to outflow disease. The patient underwent endovascular therapy with successful revascularization of his native right SFA. A stent graft was placed and good two-vessel runoff to his right foot was noted.
Two weeks later, the patient came to the office for staple removal. The incisions had closed, but he had a small seroma at the right medial thigh incision site, which dehisced the next day (Figure 1). The dehiscence was treated initially with wet to dry dressings for four weeks, but did not significantly reduce in size. At this point, due to the slow closure progression advanced skin substitute therapy was warranted. The physician felt that the patient met the medical necessity criteria for advanced skin substitute therapy and EPIFIX was investigated as an option for in-office treatments. All prior standard of care (SOC) treatments and the progression of the defect were fully documented, an insurance authorization request was submitted, and EPIFIX was approved for treatments.
EPIFIX is a dehydrated human amnion/chorion membrane allograft. EPIFIX sheets provide a protective barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic wounds. EPIFIX provides a biocompatible human extracellular matrix and contains 300+ regulatory proteins.2-4
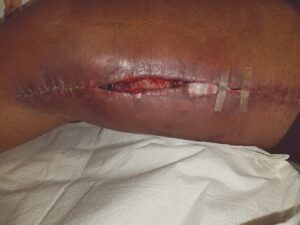 Figure 1: Dehiscence and seroma at the right medial thigh incision site |
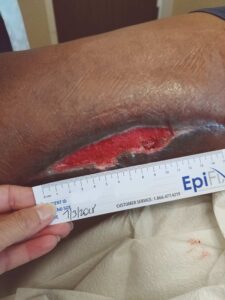 Figure 2: s/p four weeks of SOC dressings, prior to EPIFIX application |
A small area was debrided around a thrombosed vein, which was excised. A 4 cm x 4.5 cm EPIFIX Mesh allograft was cut into two pieces, placed to fit onto the wound, and dressed with a non-adherent dressing and NPWT. The NPWT dressing was left in place for five days in order to leave the EPIFIX undisturbed, and the process was repeated again the following week (Figures 2,3). Weekly applications of EPIFIX covered with non-adherent and conventional dressings were done in the office setting thereafter. The wound was closed after six weeks of EPIFIX application (Figures 4,5).
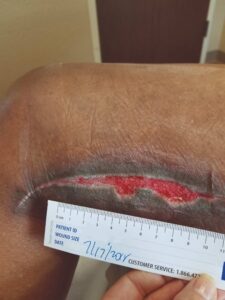 Figure 3: Week 2, s/p two EPIFIX + NPWT treatments |
 Figure 4: Week 2, s/p two EPIFIX + NPWT treatments |
Follow-Up
The patient returned for another follow-up four weeks later and the wound remained fully closed and stable (Figure 6). Lastly, the original complication with the right first toe nearly resolved with only a small scab and an amputation being avoided.
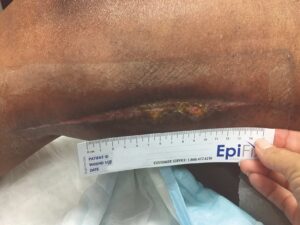 Figure 5: Week 6, s/p six EPIFIX treatments |
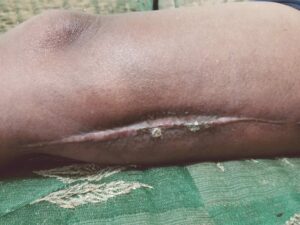 Figure 6: Week 10, wound fully closed and stable |
Conclusion
Surgical Product Portfolio: Advanced Placental-Based Allografts
MIMEDX offers a portfolio of advanced placental-based allografts in the surgical setting.
| AMNIOFIX | AXIOFILL | AMNIOCORD | AMNIOEFFECT | |
| Complex Soft-Tissue Deficit | ✓ | ✓ | ✓ | ✓ |
| Large Area Coverage | ✓ | ✓ | ✓ | |
| Affix Product with Suture* | ✓ | ✓ | ||
| Product Thickness Desired | ✓ | ✓ | ✓ | |
| Exposed Bone, Tendon, or Hardware | ✓ | ✓ | ✓ | ✓ |
| Reposition Product after Hydration | ✓ | ✓ | ✓ | ✓ |
| Minimally Invasive Procedures | ✓ | ✓ | ✓ | |
| Fenestrated Configuration to Allow Transfer of Exudate | ✓ |
References
- Atkin L, Bućko Z, Conde Montero E, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;23(Sup3a):S1-S50.
- Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
- Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
- MIMEDX Internal Report. MM-RD-00086, Proteome Characterization of PURION Processed Dehydrated Human Amnion Chorion Membrane (dHACM) and PURION PLUS Processed Dehydrated Human Umbilical Cord (dHUC) Allografts.
- Bullard JD, Lei J, Lim JJ, Massee M, Fallon AM, Koob TJ. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J Biomed Mater Res B Appl Biomater. 2019;107(4):1035-1046.
- MIMEDX Internal Report. MM-RD-00113, Non-GLP Evaluation of Placental Based Products for Cellular Response in a Mouse Subcutaneous Implant.
- Shonak B Patel, MD and Christopher J Lecroy, MD, Vascular Surgeons, Pensacola, FL.
- Ammendola M, Sacco R, Butrico L, Sammarco G, de Franciscis S, Serra R. The care of transmetatarsal amputation in diabetic foot gangrene. Int Wound J. 2017;14(1):9-15.
- Thorud JC, Jupiter DC, Lorenzana J, Nguyen TT, Shibuya N. Reoperation and Reamputation After Transmetatarsal Amputation: A Systematic Review and Meta-Analysis. J Foot Ankle Surg. 2016;55(5):1007-1012.
- Zhang JQ, Curran T, McCallum JC, et al. Risk factors for readmission after lower extremity bypass in the American College of Surgeons National Surgery Quality Improvement Program. J Vasc Surg. 2014;59(5):1331-1339.