What is HELIOGEN?
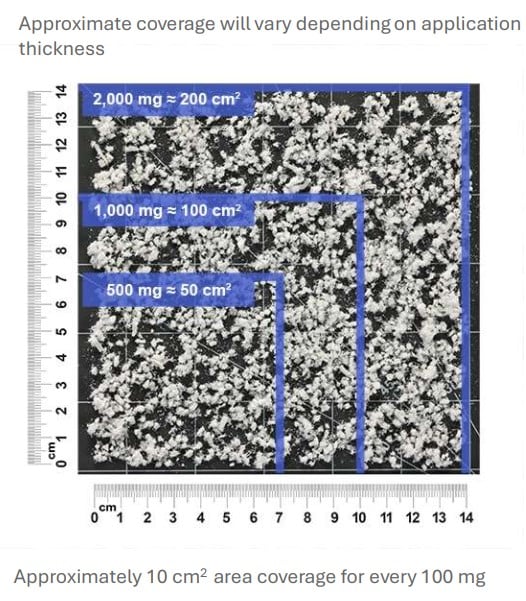
HELIOGEN is an advanced bovine collagen matrix containing type I and type III collagen. As a particulate, HELIOGEN is easy to apply dry or to hydrate with saline to create a dough, paste, or slurry consistency to address a variety of clinical needs.
Would you like to be one of the first to try it?
A Product for Complex Wounds and Minor Bleeding
HELIOGEN is intended for the management of moderately to heavily exudating wounds and to control minor bleeding. The product may be used for the management of exudating wounds such as pressure ulcers, venous stasis ulcers, diabetic ulcers, acute wounds, such as trauma and surgical wounds, and partial-thickness burns.
Clinical Use Examples
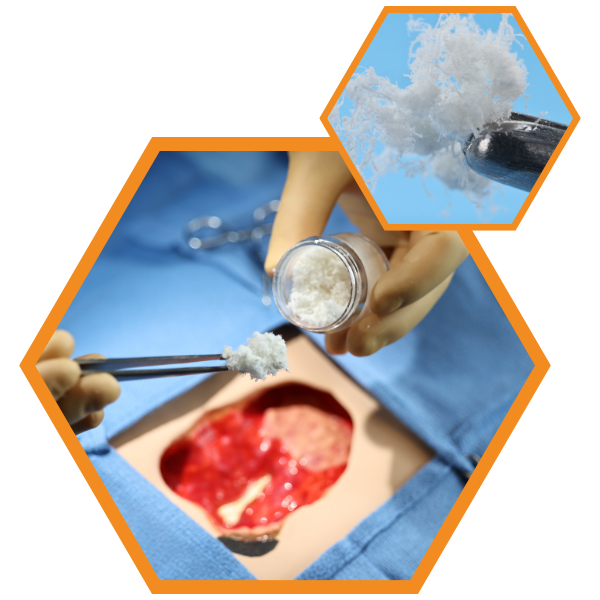
HELIOGEN’s handling properties make it versatile for applying to complex wounds in its dry form, or as a dough, paste, or slurry. Clinical use examples may include:
Dry Applications
- Surgical debridement
- Partial and full thickness wounds
- Diabetic Foot Ulcers (DFUs)
- Venous Leg Ulcers (VLUs)
- Pressure Ulcers (PUs)
- Chronic vascular ulcers
- Trauma wounds (abrasions, lacerations, burns, and skin tears)
- Draining wounds

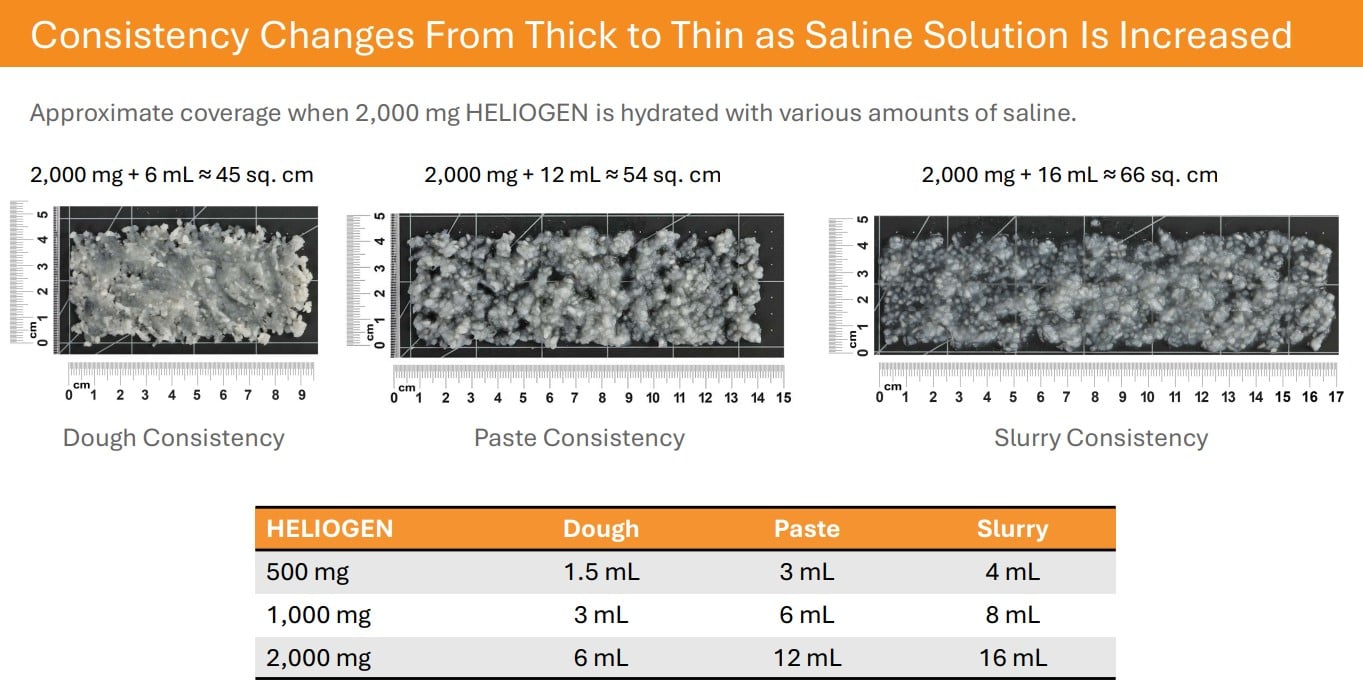
Hydrated/ Paste Applications
- Tunnel/undermined wounds
- Fistulae
- Deep, irregular wounds
- Incisions
- Surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence)

Product Advantages
- Contains type I and type III collagen
- Provides a collagen matrix known to sequester excess proteases away from the wound bed²
- Contains intrinsic hemostatic properties to control minor bleeding³⁻⁴
- Absorbency allows for the management of wound fluids while maintaining a moist environment for optimal healing³
- May be applied dry or as a hydrated paste
HELIOGEN Supports Cellular Ingrowth, Tissue Remodeling, and Resorption Over Time
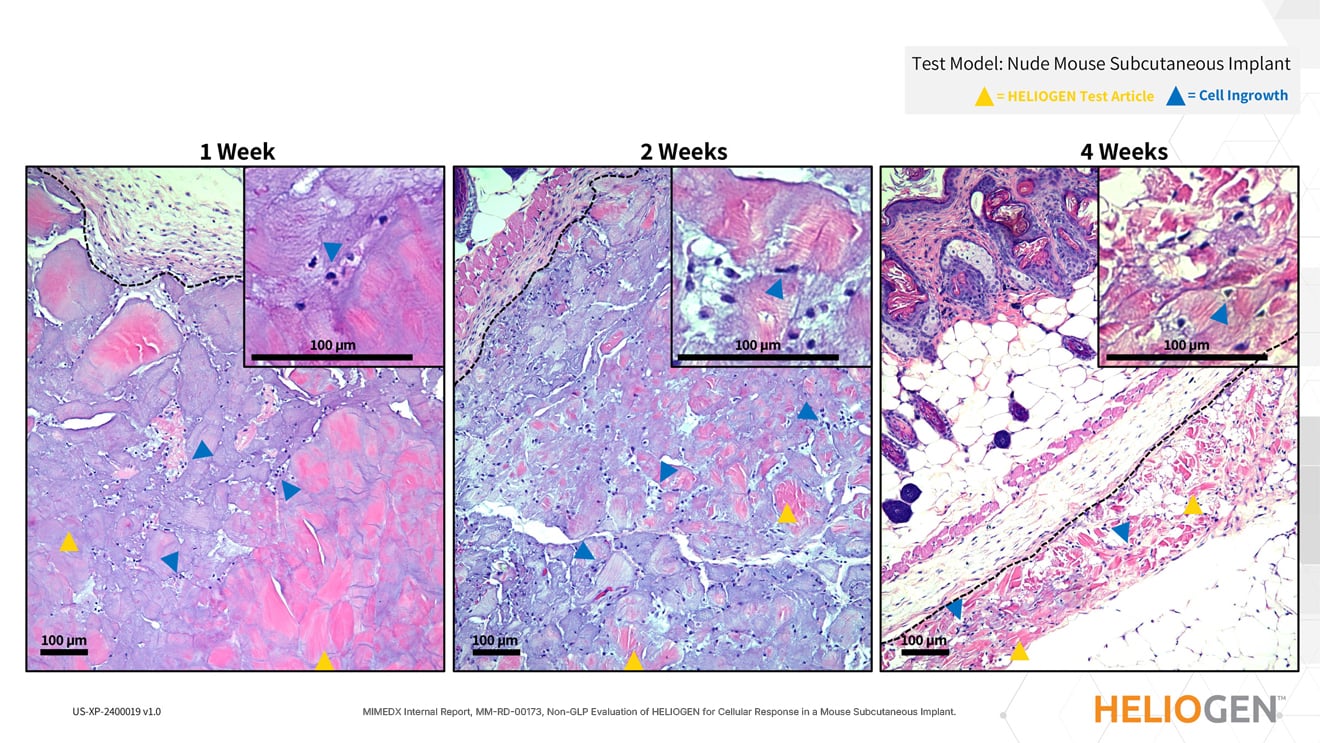
INTENDED USE: HELIOGEN Fibrillar Collagen Matrix is indicated for the management of moderately to heavily exudating wounds and to control minor bleeding. HELIOGEN Fibrillar Collagen Matrix may be used for the management of exudating wounds such as pressure ulcers, venous stasis ulcers, diabetic ulcers, acute wounds, for example trauma and surgical wounds, and partial-thickness burns.
CAUTION: Federal (US) law restricts this device to sale by or on order of a physician. Do not use HELIOGEN in patients known to be sensitive to materials of Bovine (cow) origin. Use of HELIOGEN in this patient population may result in anallergic or immunological reaction. Some reactions such as transitory pain, bleeding, blistering, swelling and redness have been reported in isolated cases using a similar product. For additional important safety information, please see the HELIOGEN Instructions for Use. Healthcare professionals must use their own clinical judgment in evaluating appropriate treatment options for a particular patient. Treatment of a specific patient should be based on individual needs and the medical care deemed necessary by the patient’s treating physician and institutional protocols. Always refer to the package insert, product label, and/or instructions for use before using any MIMEDX product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your MIMEDX representative if you have questions about MIMEDX products.
REFERENCES: 1. Regenity Internal Document. Data on File. 2. Gould LJ. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care (New Rochelle). 2016;5(1):19-31. 3. Li ST. Biologic biomaterials: tissue-derived biomaterials (collagen). In: Wong JY, Bronzino JD, ed. Biomaterials. Boca Raton: CRC Press; 2007:1-23. 4. Jaffe R, Deykin D. Evidence for a structural requirement for the aggregation of platelets by collagen. J Clin Invest. 1974;53(3):875-883.