Solutions in Burn Treatments
Advanced Placental-Based Allografts When Patients Are in Need
Burns are a major source of injury that, in the absence of care, can lead to lifelong functional loss and disfigurement. While split thickness skin autografts are the current standard of care for deep partial and full-thickness burns, this approach is associated with considerable complications. AMNIOBURN®, an advanced membrane allograft, provides an alternative with strong clinical and scientific evidence for the treatment of burns.
Video Testimonials
Potential Areas of Use:
- Points of articulation
- Head/face
- Hands
- Genitals
- Bone & Tendon
- Feet
AMNIONBURN Advantages:
- Provides a protective barrier and environment that supports the healing cascade
- Protects the wound bed to aid in the development of granulation tissue
- Provides a human biocompatible extracellular matrix (ECM) and contains 300+ regulatory proteins1-3
- Shelf-stable* with a 5-year shelf life
- Compatible with negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy (HBOT)
*See instructions for use
Case Studies:
How Physicians Use Our Products
Case study 1
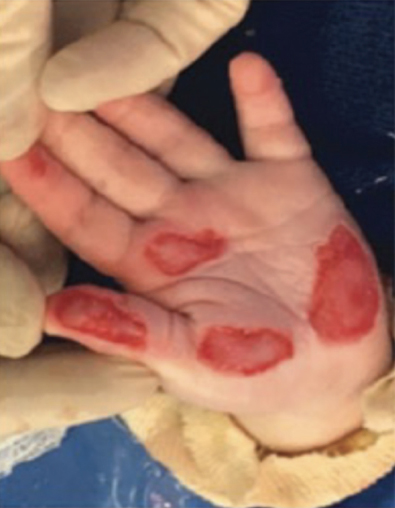
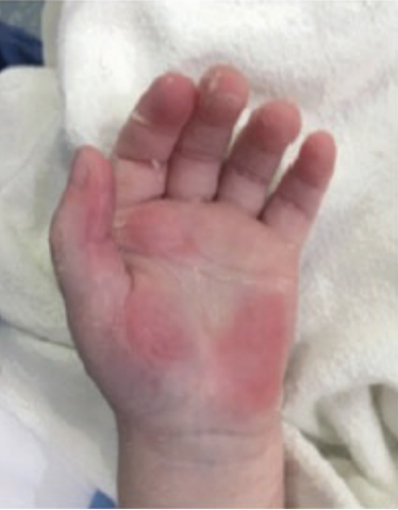
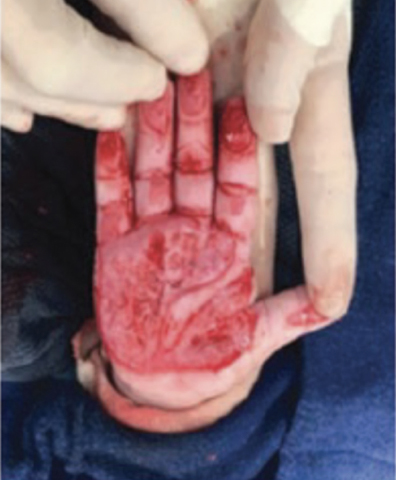
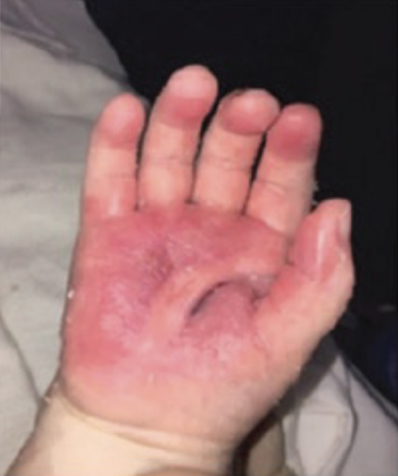
Full-Thickness Hand Burns to Fingers Bilaterally Treated With DHACM*4
|
Full-thickness hand burns to fingers bilaterally initial debridement |
Full-thickness hand burns to fingers bilaterally complete healing postop day 38 |
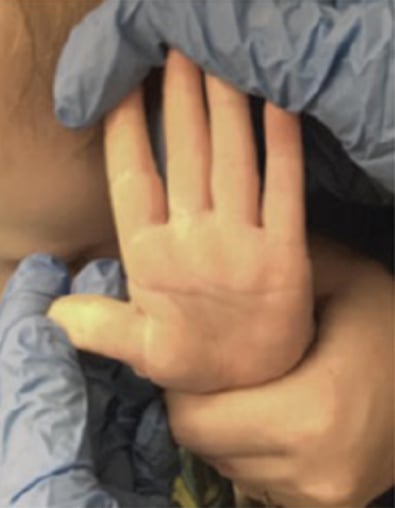 Full-thickness hand burns to fingers bilaterally postop day 115 with no contractures and minimal scarring Full-thickness hand burns to fingers bilaterally postop day 115 with no contractures and minimal scarring |
|
Full-thickness hand burns to fingers bilaterally initial debridement |
Full-thickness hand burns to fingers bilaterally complete healing postop day 38 |
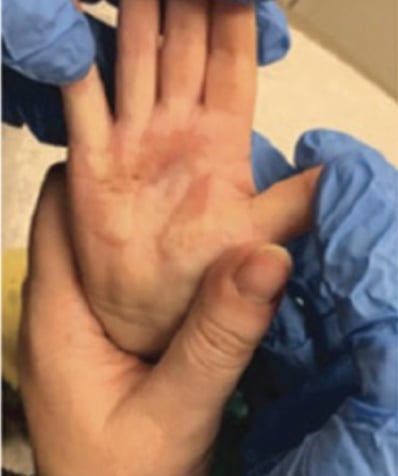 Full-thickness hand burns to fingers bilaterally postop day 115 with no contractures and minimal scarring Full-thickness hand burns to fingers bilaterally postop day 115 with no contractures and minimal scarring |
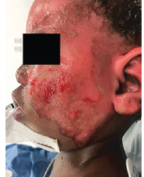
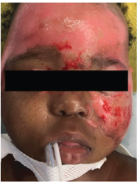
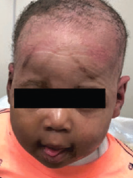
Case study 2
Superficial Partial-Thickness Facial Burn Treated with dHACM*4
|
Initial debridement (left lateral) |
Initial debridement (anterior) |
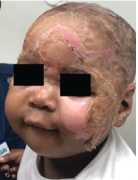 Postop Day 7 status-post DHACM application |
Complete closure |
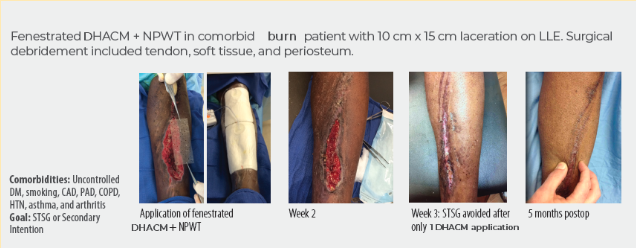
Case study 3
Full-Thickness Burn to Limb Treated With Fenestrated DHACM**+ NPWT5
Surgical Product Portfolio: Advanced Placental-Based Allografts
MIMEDX offers a portfolio of advanced placental-based allografts in the surgical setting.
| AMNIOFIX | AMNIOBURN | AMNIOCORD | AXIOFILL | AMNIOEFFECT | |
| Complex Soft-Tissue Deficit | ✓ | ✓ | ✓ | ✓ | ✓ |
| Large Area Coverage | ✓ | ✓ | ✓ | ✓ | |
| Affix Product with Suture* | ✓ | ✓ | |||
| Product Thickness Desired | ✓ | ✓ | ✓ | ||
| Exposed Bone, Tendon, or Hardware | ✓ | ✓ | ✓ | ✓ | |
| Reposition Product after Hydration | ✓ | ✓ | ✓ | ✓ | ✓ |
| Minimally Invasive Procedures | ✓ | ✓ | ✓ | ||
| Fenestrated Configuration to Allow Transfer of Exudate | ✓ |
Resources
Burn Casebook and Peer-Reviewed Publication Abstract
References
- Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
- Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
- MIMEDX Internal Report. MM-RD-00086, Proteome Characterization of PURION Processed Dehydrated Human Amnion Chorion Membrane (dHACM) and PURION PLUS Processed Dehydrated Human Umbilical Cord (dHUC) Allografts.
- Natasha Ahuja, Richard Jin, Colin Powers, Alexandria Billi, and Kathryn Bass, Pediatric Surgeons, Buffalo, NY.
- W. Dotie Jackson, MD, Plastic and Reconstructive Surgery, Jackson, MS.