Solutions in Foot & Ankle Surgery
Advanced Placental-Based Allografts When Patients Are in Need
Providing Protective Barriers and Environments that Support the Healing Cascade
Podiatric surgical procedures may need an advanced intervention to facilitate normalization of the healing cascade. MIMEDX offers an array of advanced placental-based allografts that may be used in a variety of surgical applications to provide valuable benefits in patient care.
Case Report: Exposed Tendon with AMNIOCORD®
Clinical Use Examples:
- Surgical debridement
- Diabetic and venous leg ulcers
- Tendon repair
- Amputations
- Exposed bone
- Complex incisions and dehiscence
Product Advantages: AMNIOEFFECT, AMNIOFIX, and AMNIOCORD
- Provide a protective barrier and environment that supports the healing cascade
- Protect the wound bed to aid in the development of granulation tissue
- Provide a human biocompatible extracellular matrix (ECM) and contain 250+ regulatory proteins1-4
AXIOFILL
- A lyophilized, biocompatible, and human extracellular matrix derived from the placental disc.
- For use in the replacement or supplementation of damaged or inadequate integumental tissue.
- Versatile and easy to use: apply dry or hydrated as a paste.
- Early scientific data in a nude mouse model shows AXIOFILL supports site appropriate function in tissue by allowing cell attachment, new collagen formation on the matrix, and new blood vessel formation.5
Case Studies
Case study 1
Novel Approach to Limb Salvage Utilizing Coverage of Exposed Tendon With AMNIOCORD6
Clinical History
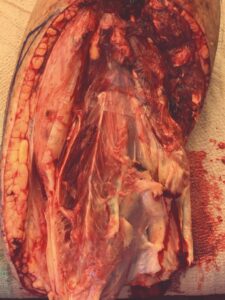
A male patient with prior medical history of alcoholic neuropathy and severe peripheral arterial disease (PAD) presented to the Emergency Department with a 10 cm x 6.5 cm wound with an exposed and desiccated tibialis anterior tendon (Figure 1). The patient had failed conservative care by a home care provider. The severity of the wound required immediate attention to salvage the limb.
Surgical Intervention
The patient was taken to the OR for debridement of the necrotic tissue and tibialis anterior tendon (Figure 2). In doing so, multiple structures were left exposed and required coverage that included the neurovascular bundle, anterior tibial artery, and dorsiflexory tendons. Two AMNIOCORD allografts were fenestrated (Figures 3) with 15 blade on the back table in order to allow for expansion and greater area coverage. AMNIOCORD grafts were secured in place with staples and dressed with negative pressure wound therapy (NPWT).
AMNIOCORD is a dehydrated human umbilical cord allograft that provides a protective environment to support the healing process. It protects the wound bed to aid in the development of granulation tissue. The product provides a human biocompatible extracellular matrix of hyaluronic acid and collagen and contains 250+ regulatory proteins.1,2
Follow-Up
Patient had follow-up visits at the wound care center and showed good wound progression (Figures 4-7). He was taken back to surgery one time after the initial consult for additional debridement. The patient expired prior to the complete closure of the wound.
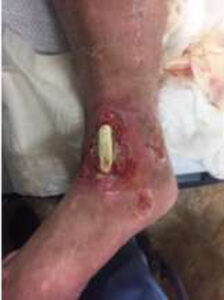 Figure 1: Day 0 presentation with exposed and desiccated tibialis anterior (TA) tendon |
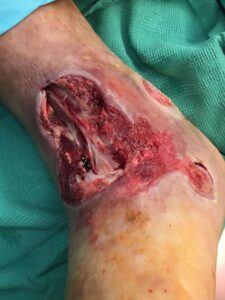 Figure 2: Day 0 debridement |
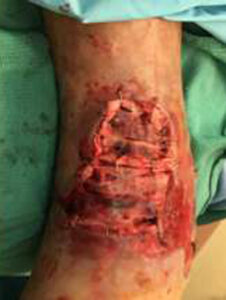 Figure 3: Day 0 two AMNIOCORD allografts placed intraoperatively |
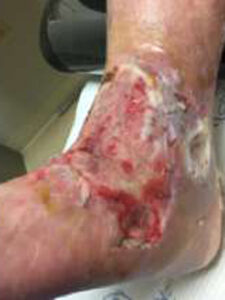 Figure 4: 4 Days postop |
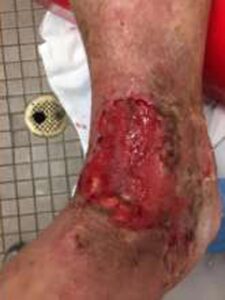 Figure 5: 2 Weeks postop Figure 5: 2 Weeks postop |
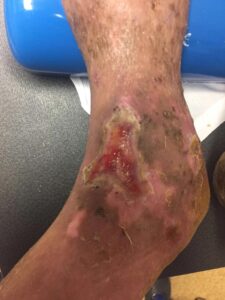 Figure 6: 4 Months postop Figure 6: 4 Months postop |
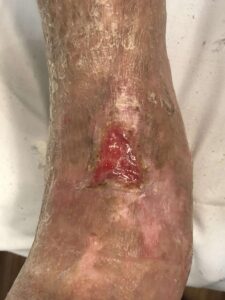 Figure 7: 6 Months postop Figure 7: 6 Months postop |
Case study 2
3-Year Chronic Venous Stasis Ulcer with DHACM7
Challenge
Wound closure is achieved through a precise sequence of three phases: inflammation, proliferation, and remodeling. All three phases must occur in the proper sequence and timeframe. Chronic wounds do not progress through the healing cascade in a timely manner. While underlying pathology will differ in chronic wounds, some commonalities of these wounds include prolonged or excessive inflammation, persistent infections, formation of drug-resistant microbial biofilms, and the inability of dermal and/or epidermal cells to respond to reparative stimuli. The combination can result in the failure of these wounds to close for prolonged periods, with high recurrence rates.8
Clinical History
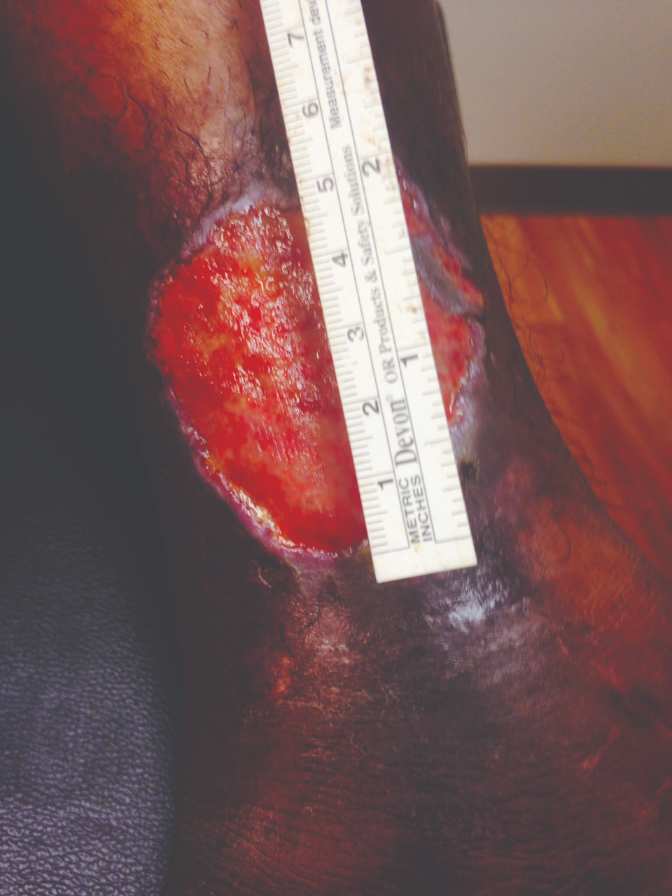
40-year-old female, status post open reduction and internal fixation surgery of an open left ankle fracture, four years prior, presents with a three-year history of a non-healing, painful venous stasis ulcer on the LLE, just proximal to the medial malleolus. The patient had received treatment from multiple healthcare providers and wound care centers prior to being seen by me. After obtaining her healthcare records, it was noted that she had been treated with local wound care, topical enzymatic ointments, recombinant DNA therapy, surgical debridement, hyperbaric oxygen therapy, and myriad skin substitutes and biologic dressings. Her prior treatments including Santyl®, Regranex®, Apligraf®, Integra®, OASIS®, and PriMatrix®. She complained of intense pain. She was upset and frustrated, as this open wound had interfered with her personal life, and the weekly visits to healthcare providers interfered with her employment. Her prior workup had included rheumatology, infectious diseases, endocrine, and nutrition. Her comorbidities included well-controlled hypertension and diabetes mellitus. At the initial consultation, the wound size was approximately 5 cm x 5 cm, with a 0.7 cm leading edge (Figures 1,); it was painful and exudative.
Surgical Intervention
Incisional biopsies, wound cultures, and sharp debridement were done in the office. Given this was a chronic wound for over three years, a malignant process had to be ruled out. Negative pressure wound therapy (NPWT) was initiated until the pathology of the biopsy site and cultures of the wound were available; the biopsies and cultures were negative. Radiographs were negative of osteomyelitis. There was no hardware in the zone of the ulcer. The fracture site was well-healed.
The wound appeared to have a healthy bed of granulation tissue to support a skin graft. She underwent a STSG with application of NPWT and was monitored in the hospital for five days. On postop day five, the negative pressure dressing was removed, and the STSG had 100% take.
She was discharged to home and seen in the office weekly. Four weeks after the skin graft, there was epidermal slough, and as the weeks progressed, there was eventually complete loss of the STSG. The donor site healed without event. Local wound care was continued.
She had normal arterial flow, but venous insufficiency was documented and she had venous stents placed and was started on anticoagulation. PriMatrix® Dermal Repair Scaffold was used for six weeks with no changes in the wound (Figures 3-5), followed by OASIS® Wound Matrix for an additional eight weeks with no significant change in the status of the wound. Her body rejected 80 percent of the skin substitutes, as evidenced by not incorporating the products. A quantitative wound assay was done and found to be normal. There was not an increased amount of zinc metalloproteases found.
A second STSG was performed four months after the venous workup was completed, and the outflow was deemed suitable. The STSG and NPWT dressing was performed, and she remained as an inpatient for five days. Again, the graft was 100% viable at that time and remained so for eight weeks. The graft became unstable again and was lost. The wound was debrided in the office, and a DHACM (Dehydrated Human Amnion/Chorion Membrane) 4 cm x 4 cm graft was placed onto the wound bed and covered with an NPWT dressing. DHACM provides a barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic closures. The product is a biocompatible human extracellular matrix and contains 300+ regulatory proteins.1,3,4
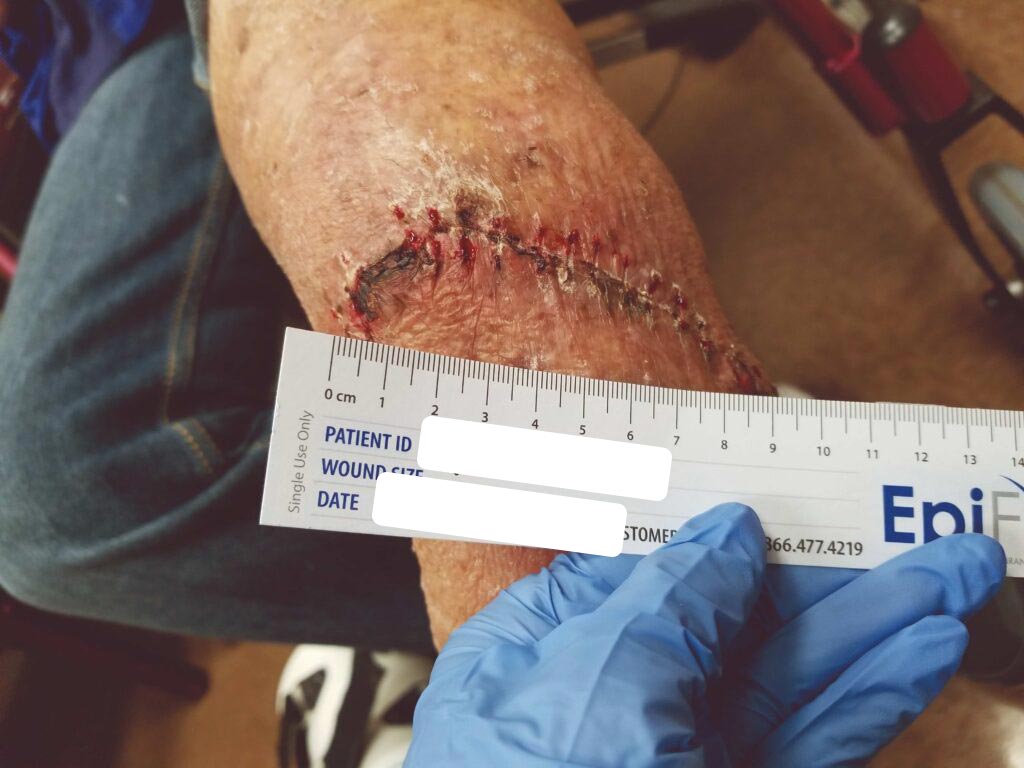
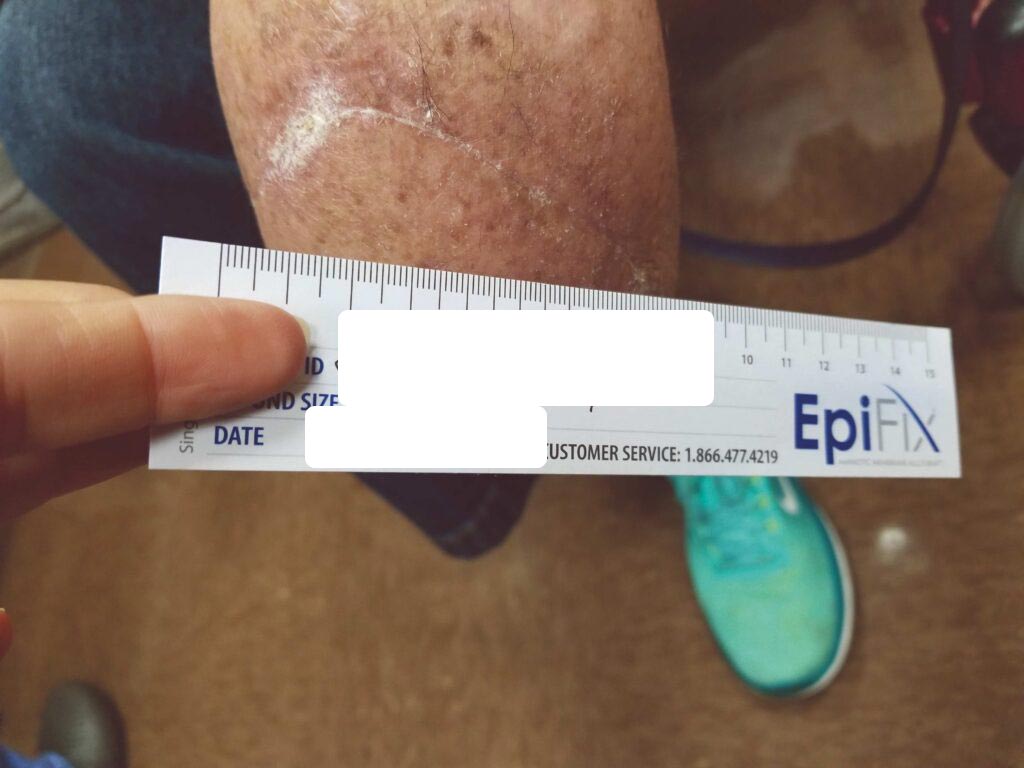
Within one week, the patient stated her pain had resolved. There was visible ulcer contraction and skin ingrowth noted at the periphery of the wound. An additional DHACM graft was placed two weeks after the initial application, and by day 35, the ulcer was closed and stable for the first time in more than four years (Figures 6-10). The skin was normal and pliable. The patient was pain free and ready to return to work.
Follow-Up
The patient has been seen at three, six, and twelve months. The skin is soft and pliable, and the patient is without complaints. After her journey, she asked me, “Where were you four years ago?”
Conclusion
Patients with multiple comorbidities and venous insufficiency can have significant healing challenges. In this case example, the patient failed multiple treatments using both conventional and advanced wound therapies and STSGs over a three-year period. Closure was observed approximately one month after DHACM and NPWT applications were initiated.
 |
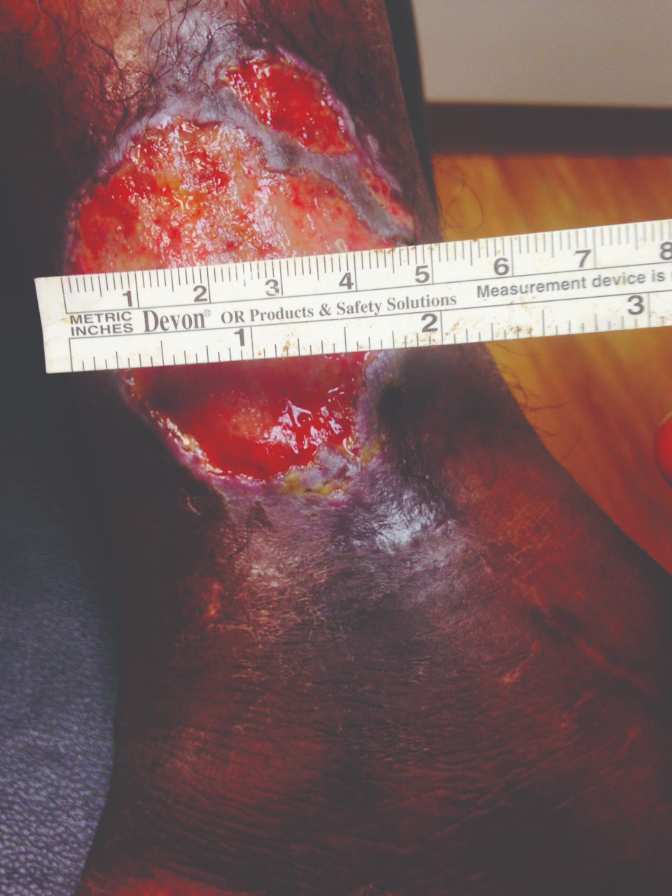 |
Figure 1 & 2: 5 cm x 5 cm chronic VLU
 Figure 3: Failed PriMatrix® |
 Figure 4: Debridement and NPWT |
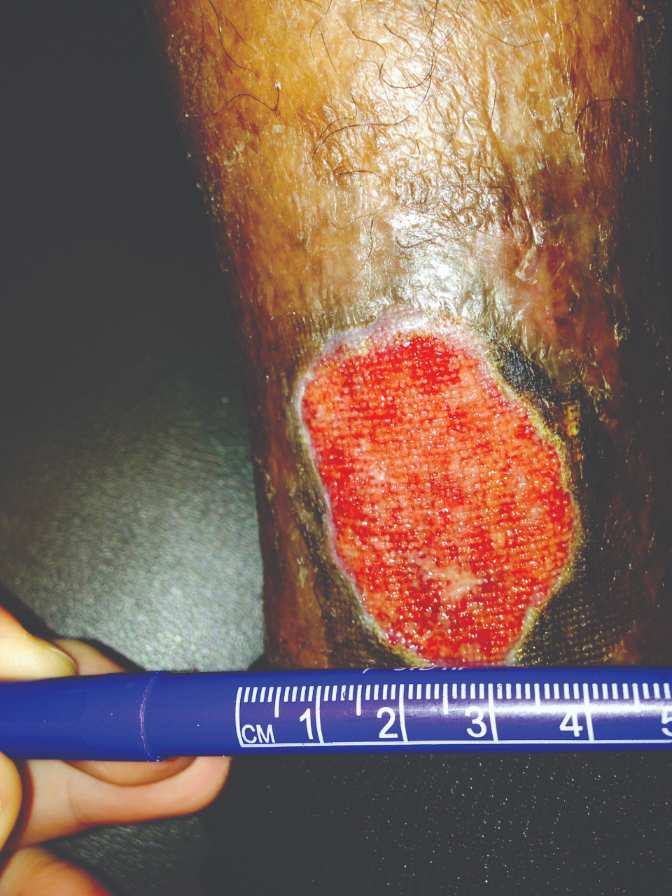 Figure 5: Removal of NPWT one week later |
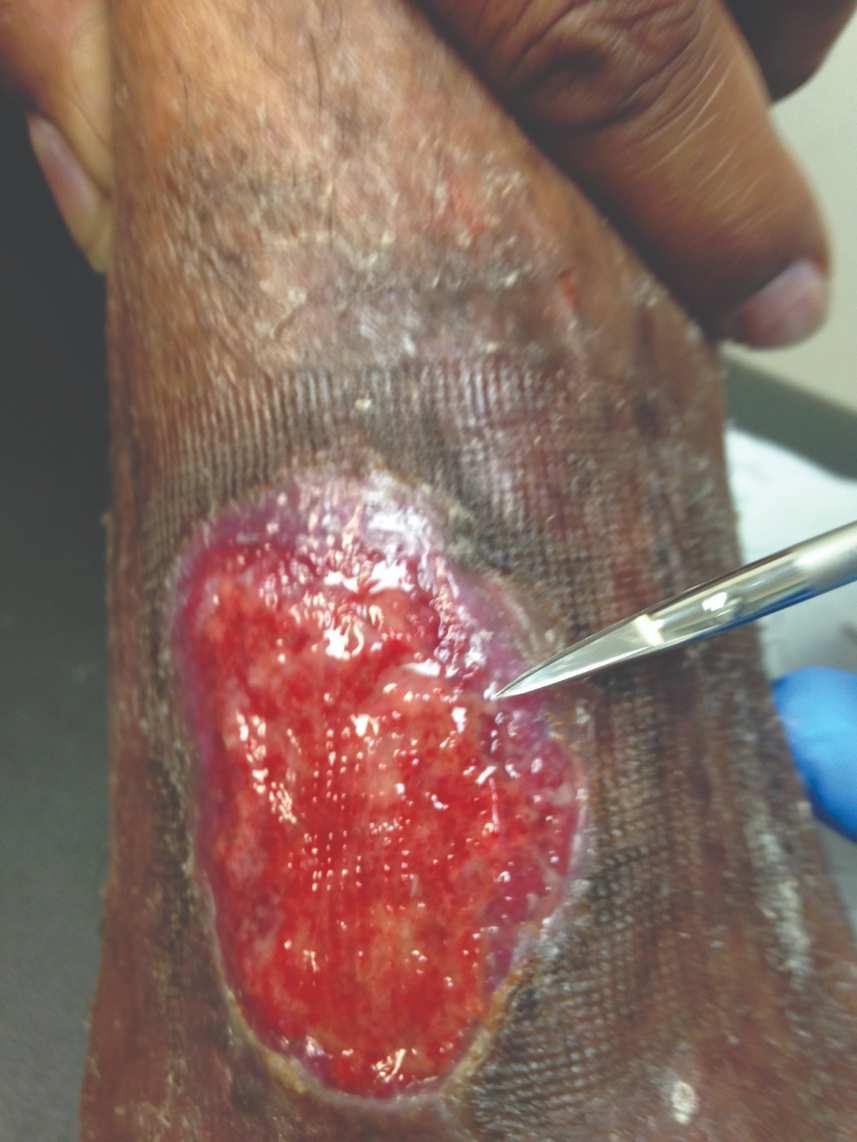 Figure 6: 7 days s/p dHACM + NPWT |
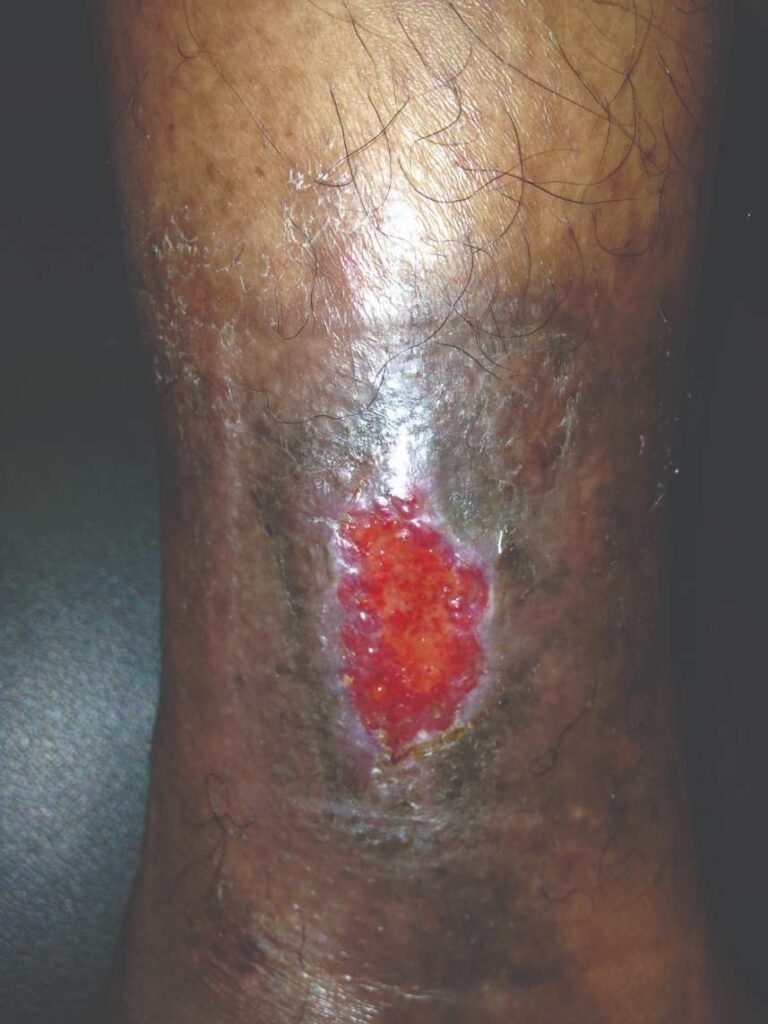 Figure 7: Day 14 |
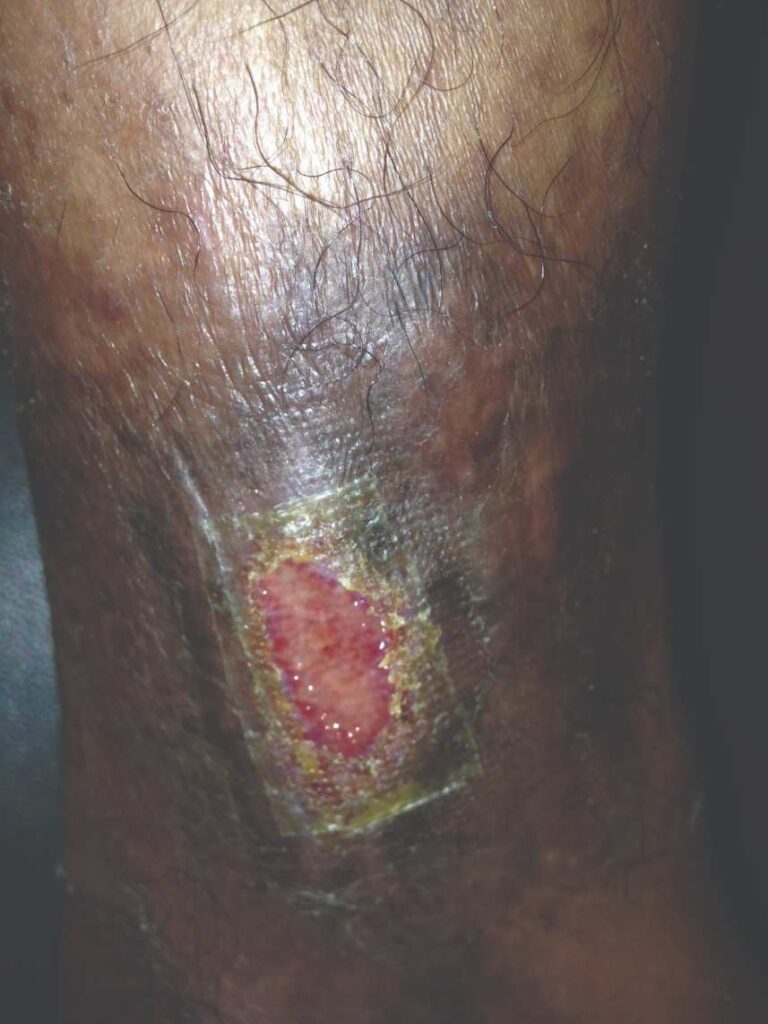 Figure 8: dHACM Figure 8: dHACMApplication on Day 14 |
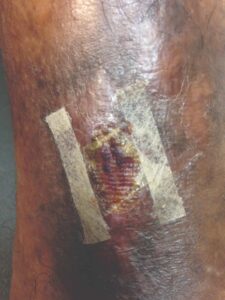 Figure 9: Day 25 |
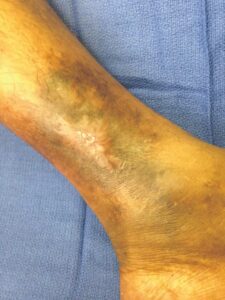 Figure 10: Day 35 |
Case study 3
BKA After Prior Amputation Dehiscence with AMNIOFIX9
Challenge
Despite advances in vascular surgery, endovascular therapy, and soft tissue management, closure of diseased limbs remains challenging. The primary goal of limb salvage is to restore and maintain ambulation. However, closure challenges can further complicate the clinician’s efforts. Challenges to wound closure in this setting include non-compliance, PAD, hygiene, smoking, multiple comorbidities, etc. Reported closure rates for transmetatarsal amputations (TMA) range from 40-70%,10 while reoperation rates range from 8 to 63%, with approximately one-third resulting in a major amputation.11 Once other more conservative limb salvage options have been exhausted, BKA can still offer the patient a relatively functional limb for use with prosthetic to ambulate.
Clinical History
The patient is a 56-year-old female with a history of severe peripheral artery disease, uncontrolled diabetes, end-stage renal disease on dialysis, CAD with a prior percutaneous coronary intervention, and diabetic foot ulcers that led to a TMA by podiatry. She did not have the appropriate arterial revascularization at the time and the TMA site dehisced due to pressure from walking and the impaired closure from severe comorbidities. The wound worsened with necrosis and infection, to the point where re-approximation of the tissue was no longer possible (Figure 1).
The patient was referred to vascular surgery for her lower limb PAD. Atherectomy and angioplasty were performed to address her superficial femoral artery stenosis and anterior tibial artery stenosis, which successfully improved perfusion to the foot with three-vessel runoff. The wound would still not heal after one month with multiple debridements and treatments by podiatry. The patient was referred back to vascular surgery and scheduled for a BKA.
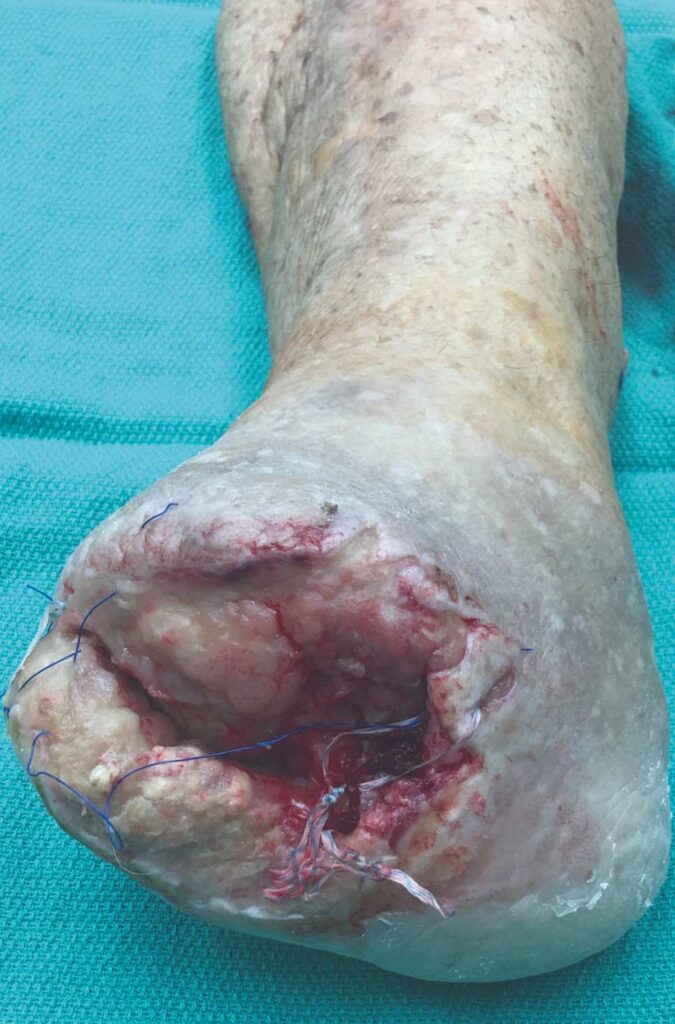 Figure 1: Non-healing dehiscence after TMA |
Surgical Intervention
A standard BKA was performed. Based on the patient’s history of an inability to close wounds in the setting of adequate perfusion, AMNIOFIX was used. AMNIOFIX is a dehydrated human amnion/chorion membrane allograft. AMNIOFIX sheets provide a protective barrier that supports the healing cascade. It also protects the wound bed to aid in the development of granulation tissue in chronic and acute wounds. AMNIOFIX provides a human biocompatible extracellular matrix and contains 300+ regulatory proteins.1,3,4
A 6 cm by 16 cm AMNIOFIX allograft was cut into several pieces and placed into the surgical site. One portion of the allograft was placed on the muscular bed, where the AMNIOFIX allograft wound be positioned between muscle and bone after the subcutaneous tissue was closed (Figure 2). The other portions were placed in the subcutaneous space before dermal closure to enhance closure in this high-risk suture line (Figures 3-5).
|
Figure 2: AMNIOFIX placed on muscular bed |
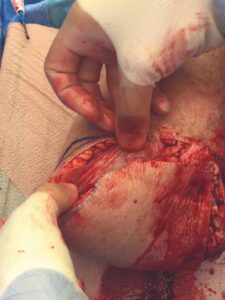 Figure 3: AMNIOFIX in subcutaneous tissue before closure Figure 3: AMNIOFIX in subcutaneous tissue before closure |
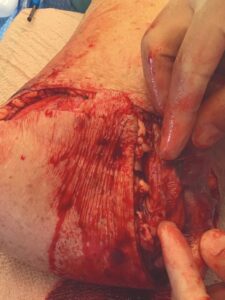 Figure 4: AMNIOFIX in subcutaneous tissue before closure Figure 4: AMNIOFIX in subcutaneous tissue before closure |
Figure 5: Flap closure |
Follow-Up
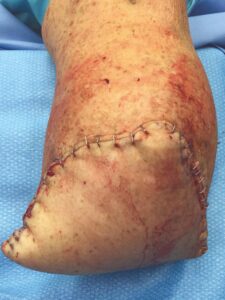
The patient fell onto her stump in the hospital five days postoperatively. Despite the fall and resultant mild dehiscence, there was no swelling and the stump remained intact (Figure 6). This type of injury could have resulted in amputation failure. After 30 days, the BKA incision was fully closed. The patient was again seen 11 weeks postop and the amputation remained stable (Figure 7).
|
Figure 6: s/p fall onto stump on Day 5 |
Figure 7: Wound fully closed |
Conclusion
Resources
Surgical Lower Extremity Casebook
Surgical Product Portfolio: Advanced Placental-Based Allografts
- AMNIOFIX®
- AMNIOCORD®
- AMNIOEFFECT®
- AMNIOBURN®
- AXIOFILL®
References
- MIMEDX Internal Report. MM-RD-00086, Proteome Characterization of PURION Processed Dehydrated Human Amnion Chorion Membrane (dHACM) and PURION PLUS Processed Dehydrated Human Umbilical Cord (dHUC) Allografts.
- Bullard JD, Lei J, Lim JJ, Massee M, Fallon AM, Koob TJ. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J Biomed Mater Res B Appl Biomater. 2019;107(4):1035-1046.
- Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
- Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/ chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
- MIMEDX Internal Report. MM-RD-00113, Non-GLP Evaluation of Placental Based Products for Cellular Response in a Mouse Subcutaneous Implant.
- David Kyle, DPM, Podiatric Surgeon, Huntsville, AL.
- W. Dotie Jackson, MD, Plastic and Reconstructive Surgery, Jackson, MS.
- Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4(9):560-582.
- Shonak B Patel, MD and Christopher J Lecroy, MD, Vascular Surgeons, Pensacola, FL.
- Ammendola M, Sacco R, Butrico L, Sammarco G, de Franciscis S, Serra R. The care of transmetatarsal amputation in diabetic foot gangrene. Int Wound J. 2017;14(1):9-15.
- Thorud JC, Jupiter DC, Lorenzana J, Nguyen TT, Shibuya N. Reoperation and reamputation after transmetatarsal amputation: a systematic review and meta-analysis. J Foot Ankle Surg. 2016;55(5):1007-1012.