Solutions in Plastic Surgery
Advanced Placental-Based Allografts When Patients Are in Need
Providing Protective Barriers and Environments that Support the Healing Cascade
There are many risk-factors to healing such as patient co-morbidities and complex defects.1 MIMEDX offers an array of advanced placental-based allografts that may be used in a variety of surgical applications to provide valuable benefits in patient care.
Product Advantages: AMNIOEFFECT, AMNIOFIX, and AMNIOCORD
- Provide a protective barrier and environment that supports the healing cascade
- Protect the wound bed to aid in the development of granulation tissue
- Provide a human biocompatible extracellular matrix (ECM) and contain 250+ regulatory proteins2-5
AXIOFILL:
- A lyophilized, biocompatible, and human extracellular matrix derived from the placental disc.
- For use in the replacement or supplementation of damaged or inadequate integumental tissue.
- Versatile and easy to use: apply dry or hydrated as a paste.
- Early scientific data in a nude mouse model shows AXIOFILL supports site appropriate function in tissue by allowing cell attachment, new collagen formation on the matrix, and new blood vessel formation.6
Clinical Use Examples:
- Acute and chronic wounds
- Debridements
- Exposed bone and tendon
- Dehiscence
- Decubitus ulcers
- Flaps
- Bridge to STSG
- Limb salvage
Case Studies:
How Physicians Use Our Products
Case study 1
Acute Abdominal Wall Dehiscence With EPIFIX7
Challenge
62-year-old obese male, BMI of 29, type II diabetes, with a history of hypertension, myocardial infarction with stent placements, multiple abdominal surgeries, and over forty years of cigarette smoking, underwent large ventral hernia repair. At one week post op, the patient developed ischemia at the incision line, which led to an incisional dehiscence.
Studies have shown a direct correlation between the number of comorbidities and clinical outcomes. A significant rise in complications, length of stay, and mortality rates is associated with the rise in number of patient comorbidities.8-10
Surgical Intervention
The patient was managed with serial debridement and wet-to-dry dressings for two months, then placed on negative pressure wound therapy (NPWT) for four weeks at home. After one month of NPWT, the wound had only decreased by 30%. NPWT was discontinued, and DHACM (Dehydrated Human Amnion/Chorion Membrane) was applied every other week, instead of weekly, due to the travel distance for the patient. The product provides a barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic closures. It provides a biocompatible human extracellular matrix and contains 300+ regulatory proteins.2-4
Follow-Up
Upon examination at his two month follow-up visit, the wound was fully closed and re-epithelialized. The patient returned for a routine one-year visit and has remained fully closed and asymptomatic.
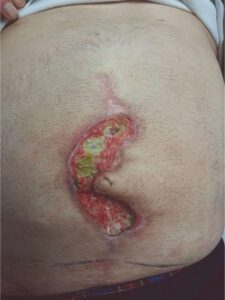 Following Debridement Following Debridement |
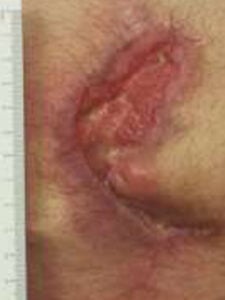 Four weeks of NPWT, only 30% size reduction, first DHACM 4 cm x 4 cm applied Four weeks of NPWT, only 30% size reduction, first DHACM 4 cm x 4 cm applied |
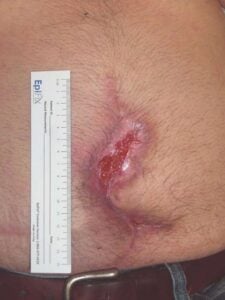 Week 2: Two 2 cm x 3 cm DHACM applied Week 2: Two 2 cm x 3 cm DHACM applied |
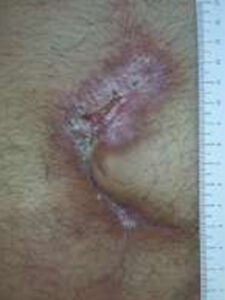 Week 4: One 2cm x 3 cm DHACM applied Week 4: One 2cm x 3 cm DHACM applied |
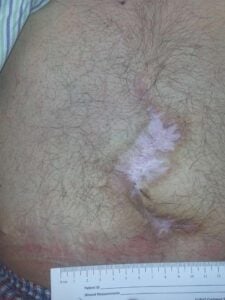 Week 8: Wound closed and stable Week 8: Wound closed and stable |
Case Study 2
Keloid Scar Revision With EPIFIX11
Clinical History
Patient presented with keloid after Caesarean section procedure.
Treatment
One third of the keloid scar was treated with DHACM (Dehydrated Human Amnion/Chorion Membrane) in revision surgery to evaluate its outcome prior to treating the remainder of the scar. DHACM was placed within the incision site before suturing.
DHACM provides a barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic closures. It provides a biocompatible human extracellular matrix and contains 300+ regulatory proteins.2-4
Follow-Up
The scar was greatly reduced in height and in color. Subsequent revision surgery treated the remainder of the keloid scar with DHACM.
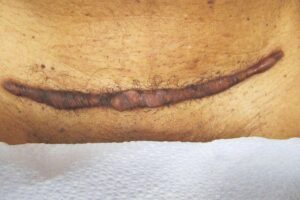 KELOID Scar Revision with DHACM KELOID Scar Revision with DHACM |
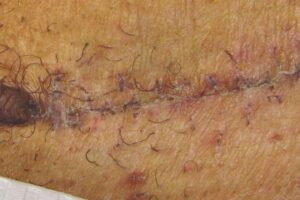 Post-scar revision using DHACM on 1/3 portion of original scar Post-scar revision using DHACM on 1/3 portion of original scar |
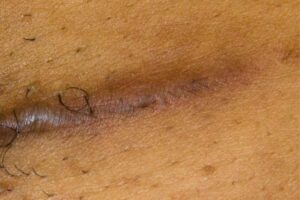 Scar one year post DHACM treatment Scar one year post DHACM treatment |
Resources
Plastic Reconstruction Casebook
Surgical Product Portfolio: Advanced Placental-Based Allografts
MIMEDX offers a portfolio of advanced placental-based allografts in the surgical setting.
| AMNIOFIX | AXIOFILL | AMNIOCORD | AMNIOEFFECT | |
| Complex Soft-Tissue Deficit | ✓ | ✓ | ✓ | ✓ |
| Large Area Coverage | ✓ | ✓ | ✓ | |
| Affix Product with Suture* | ✓ | ✓ | ||
| Product Thickness Desired | ✓ | ✓ | ||
| Exposed Bone, Tendon, or Hardware | ✓ | ✓ | ✓ | ✓ |
| Reposition Product after Hydration | ✓ | ✓ | ✓ | ✓ |
| Minimally Invasive Procedures | ✓ | ✓ | ||
| Fenestrated Configuration to Allow Transfer of Exudate | ✓ |
References