Spine and Neurosurgery
Spine revisions and cranioplasties can be complicated by long dissection time and adverse outcomes due to fibrosis from previous procedures.1,2
AMNIOFIX and AMNIOEFFECT are placental-based allografts that provide a protective barrier to support the healing cascade and contain 300+ regulatory proteins.3-6
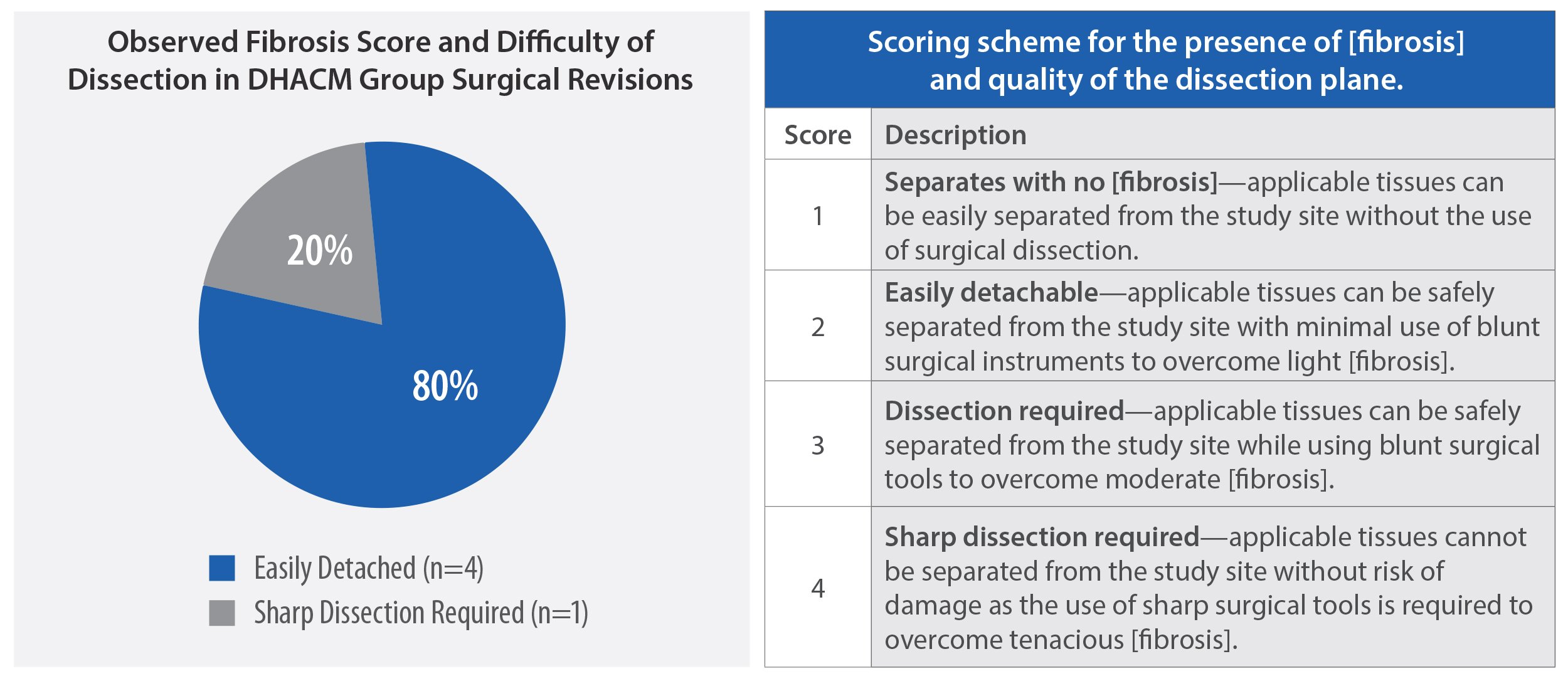
Peer-reviewed RCTs and other studies have reported favorable results in patients undergoing dural repair in cranial and spine surgeries with human amniotic membrane allografts.1,7 In one study, 80% of AMNIOFIX recipients had minimal and easily excised fibrotic tissue in spine revision procedures.1
AMNIOFIX Applications
Laminectomy & Fusion
Microdiscectomy
Craniectomy
Product Advantages: AMNIOFIX and AMNIOEFFECT
- Provides a protective barrier that supports the healing cascade
- Contains 300+ regulatory proteins3-6
Clinical Use Examples:
SPINE
- Laminectomies
- Fusions
- Revisions
- Microdiscectomies
NEUROSURGERY
- Craniectomies
- Craniotomies
- Cranioplasties
Case Studies and Clinical Evidence:
How Physicians Use Our Products
spine case study
Posterior Lateral Fusion With Decompression and Application of AMNIOFIX8
Patient Background
69-year-old male treated for neurogenic claudication for 2 years with conservative measures (epidural steroid shots) and started to develop left ankle dorsiflexion weakness.
Surgical Intervention
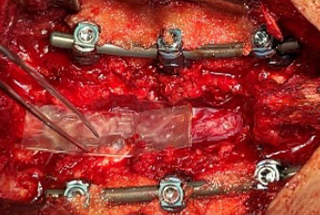
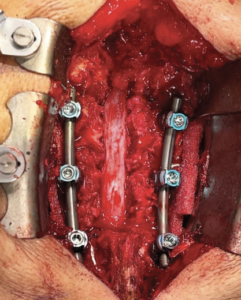
- Patient underwent a lateral interbody fusion at L3-L4 and L4-L5 and a laminectomy L3-L5 to achieve a decompression.
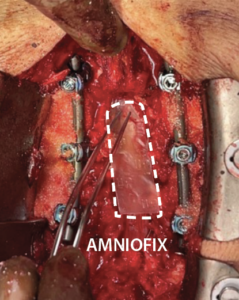
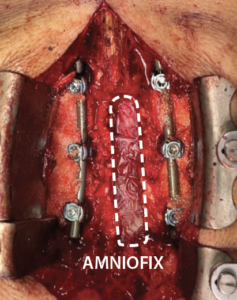
- Prior to closure, a 4 cm x 6 cm AMNIOFIX sheet was cut lengthwise into two pieces.
- The first 2 cm x 6 cm AMNIOFIX sheet was applied to the exposed dura and tucked underneath the lamina and lateral recesses.
- The second 2 cm x 6 cm AMNIOFIX sheet was applied on the cranial aspect of the exposed dura. The allograft was then tucked into the lateral gutters and underneath the cephalad lamina.
Goal
AMNIOFIX utilized to provide a protective barrier to support the healing cascade.
Conclusion
- The dura was decompressed and no postop complications were reported. The surgeon and patient were pleased with the outcome.
- The surgeon anticipated that this patient will likely have adjacent level disease to the treated area that would be symptomatic in 5 to 10 years and may require an adjacent level decompression.
|
Lateral interbody fusion L3-L4 and L4-L5 and laminectomy L3-L5. |
4 cm x 6 cm AMNIOFIX sheet was cut lengthwise into two pieces. The first 2 cm x 6 cm AMNIOFIX sheet was applied to the exposed dura and tucked underneath the lamina and lateral recesses. |
The second 2 cm x 6 cm AMNIOFIX sheet was applied on the cranial aspect of the exposed dura. Allograft was tucked into the lateral gutters and underneath the cephlad lamina. |
spine revision clinical evidence
PEER-REVIEWED STUDY
The Use of a Dehydrated Amnion/Chorion Membrane Allograft in Patients Who Subsequently Undergo Reexploration After Posterior Lumbar Instrumentation1
ABSTRACT
Background Context: Products that can reduce development of epidural fibrosis may reduce risk for ongoing pain associated with development of [fibrotic] tissue and make subsequent epidural reexploration easier.
Purpose: To evaluate the use of Dehydrated Human Amnion/Chorion Membrane (DHACM) on the formation of soft tissue [fibrosis] in the epidural space.
Study Design: Case series.
Patient Sample: Five patients having transforaminal lumbar interbody lumbar fusion (TLIF) with posterior instrumentation and implantation of DHACM in the epidural space and subsequent epidural reexploration.
Outcome Measures: Degree of [fibrotic] tissue adjacent to the epidural space at reexploration. Intraoperative and postoperative complications related to DHACM and patient reported outcomes.
Methods: [Reoperation was performed after] “radiological studies had been performed to document adequate fusion, as evidenced by bridging bone within the interbody space and adjacent intertransverse region.” The degree of fibrotic tissue adjacent to the epidural space was assessed during the reexploration surgery. Patients’ outcomes were collected using standard validated questionnaires.
Results: Four of 5 cases had easily detachable tissue during epidural reexploration. Angiolipoma of 10% was noted in 1 case and 5% in 2 cases. Significant improvements in patient reported outcomes were observed. No intraoperative or postoperative complications occurred.
Conclusions: Our findings suggest that DHACM implant during TLIF may have favorable effects on epidural fibrosis and is well-tolerated. Further studies with larger cohorts are required to prove our results.
HIGHLIGHTED RESULTS:
neuro case study
Craniotomy for Tumor Resection and Application of AMNIOEFFECT9
Clinical Background
59-year-old male presented with significant right sided upper extremity weakness and aphasia. MRI showed a large left sided mass causing symptoms and concerning malignancy.
Comorbidities
25-year history of smoking half a pack or more a day and hypertension.
Surgical Intervention
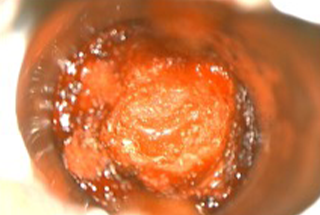
- Craniotomy was performed, tumor resected, and chemo wafers placed in resection cavity.
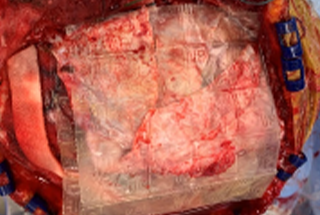
- 6 cm x 16 cm AMNIOEFFECT sheet placed on brain. Surgeon was very satisfied with the ease of handling of the product, the ability to visualize it, and that it stayed in place while using suction.
- Bone flap screwed into place with metal plating system.
- Multi-layer tissue closure on the skull.
Goal
AMNIOEFFECT utilized to provide a protective barrier to support the healing cascade.
Follow Up
- Approximately two weeks postop, cranial incision staples removed.
- Incision was intact and closed.
- Patient had improvement of both his right upper extremity strength and speech.
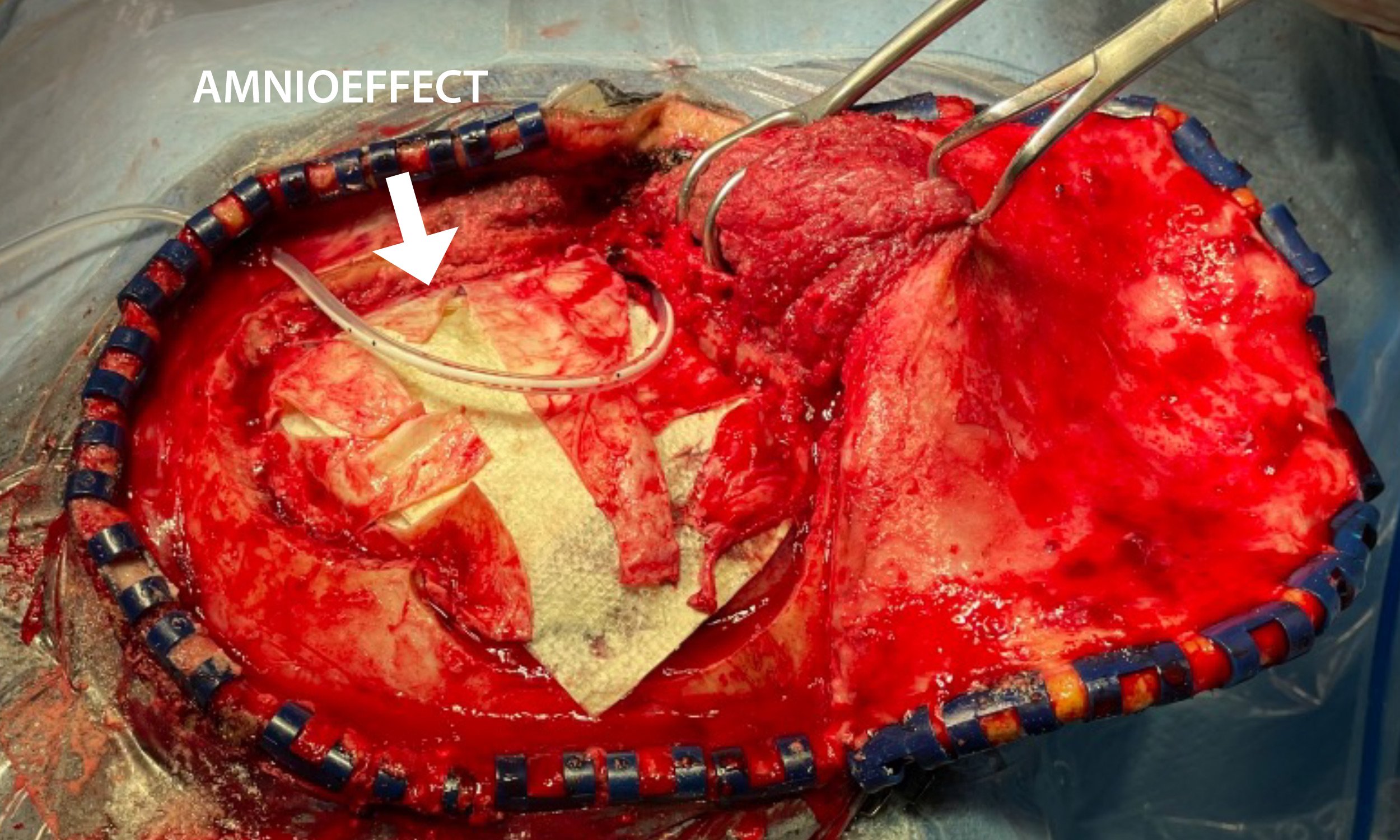
6 cm x 16 cm AMNIOEFFECT sheet placed on brain.
Resources
Spine & Neuro Casebook
Surgical Product Portfolio: Advanced Placental-Based Allografts
MIMEDX offers a portfolio of advanced placental-based allografts in the surgical setting.
- AMNIOFIX®
- AMNIOCORD®
- AMNIOEFFECT®
- AMNIOBURN®
- AXIOFILL®
References