Thyroid Surgery
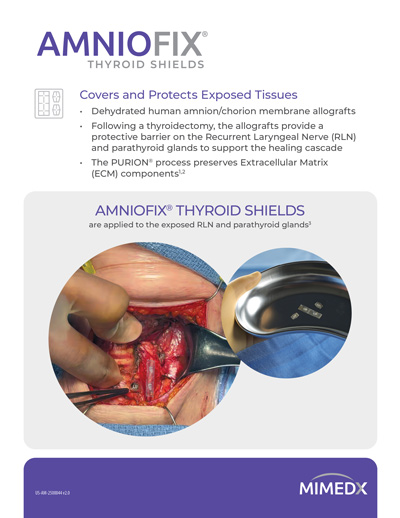
AMNIOFIX® Thyroid Shields Cover and Protect Exposed Tissues After Thyroidectomies
Endocrine surgery procedures, including thyroidectomy, may involve exposure of tissues such as the Recurrent Laryngeal Nerve (RLN) and parathyroid glands.
Following thyroidectomy, protection of the RLN and parathyroid glands is an important consideration due to their functional significance and vulnerability.
AMNIOFIX Thyroid Shields dehydrated human amnion/chorion allografts are preconfigured for use in thyroid surgery to provide a protective barrier on exposed tissues and support the healing cascade.
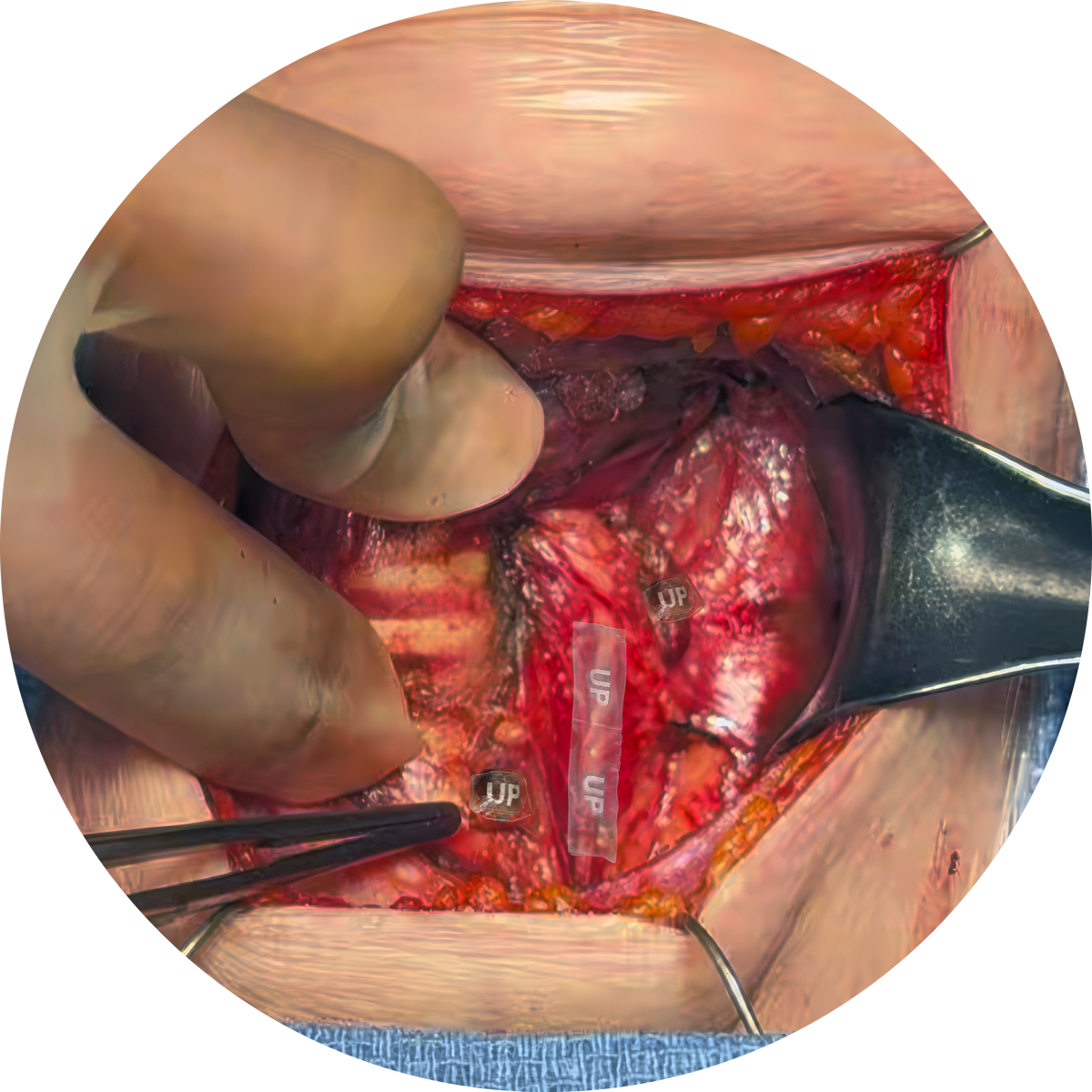

AMNIOFIX Thyroid Shields are applied to the exposed RLN and parathyroid glands
Product Advantages:
- Preconfigured sizes intended to prevent the need for intraoperative trimming, helping save valuable operating room time
- PURION® process preserves Extracellular Matrix (ECM) components, including 300+ regulatory proteins1,2
- Terminally sterilized for an additional level of safety
- Easy to apply
- Shelf stable*
- Up to 5 year shelf life
- Does not expand upon hydration3
*See Instructions for Use
Clinical Use Example:
- Thyroidectomy surgery
Clinical Evidence:
Three peer‑reviewed, single‑center prospective cohort studies with historical controls evaluating AMNIOFIX as a protective barrier on exposed RLN tissue following thyroidectomy demonstrated statistically significant positive outcomes.4-6
Clinical Summary
Clayman G, et al. J Interdiscip Histopathol 2022 | Peer-Reviewed Study4
Clinical Summary
Study Design:
- A single-center, prospective data study with historical controls compared thyroidectomy patients with and without AMNIOFIX, applied to >3cm fully dissected RLNs, as a protective barrier to support the healing cascade.
- The postop outcomes were investigated with and without AMNIOFIX as a protective barrier on RLNs and the occurrence of RLN damage.
Cohorts: The intervention and historical control groups were matched:
- AMNIOFIX (Intervention): 670 patients including 2,000 at-risk RLNs
- Non-AMNIOFIX (Historical Control): 1,420 patients including 1,000 at-risk RNLs
Methods:
- All thyroidectomies were performed by the same surgeons using nerve monitoring endotracheal tubes
- Vocal outcomes were assessed using patient-reported vocal analysis, physician-assessed vocal analysis, and laryngoscopic assessment of vocal fold dysfunction were performed before and after surgery
Results:
- 3.4% incidence of RLN damage with the AMNIOFIX group at 24 hours postop, which was a 76% reduction vs. 14.4% with the non-AMNIOFIX group (p<0.01)
Resources
AMNIOFIX Thyroid Shields Brochure – Product Information
References
- Moreno S, Massee M, Campbell S, Bara H, Koob TJ, Harper JR. PURION® processed human amnion chorion membrane allografts retain material and biological properties supportive of soft tissue repair. J Biomater Appl. 2024;39(1):24-39.
- MIMEDX Internal Report. MM-RD-00086.
- MIMEDX Internal Report. MM-RD-00193.
- Clayman G, Roy R, Walsh N. Human Amnion/Chorion Membrane Use During Neck Surgery to Protect the Recurrent Laryngeal Nerve. J Interdiscip Histopathol. 2022;10(5):1-4.
- Clayman GL, Roy R, Norman J. Human Amnion/Chorion Membrane May Reduce Transient Recurrent Laryngeal Nerve Injury During Thyroid Surgery. Cell Transplant. 2022;31:9636897211073136.
- Carling T. Protection of laryngeal nerve palsy using amniotic membrane shield during thyroid surgery. Endocrine. 2021;74(1):197‑199.