What is AXIOFILL?
AXIOFILL® is a biocompatible acellular human placental Extracellular Matrix (ECM) derived from the placental disc. It is intended for use in the replacement or supplementation of damaged or inadequate integumental tissue. Clinical use examples include deep, tunneling, or irregular soft tissue deficits and incision management in surgical applications.
AXIOFILL Product Benefits
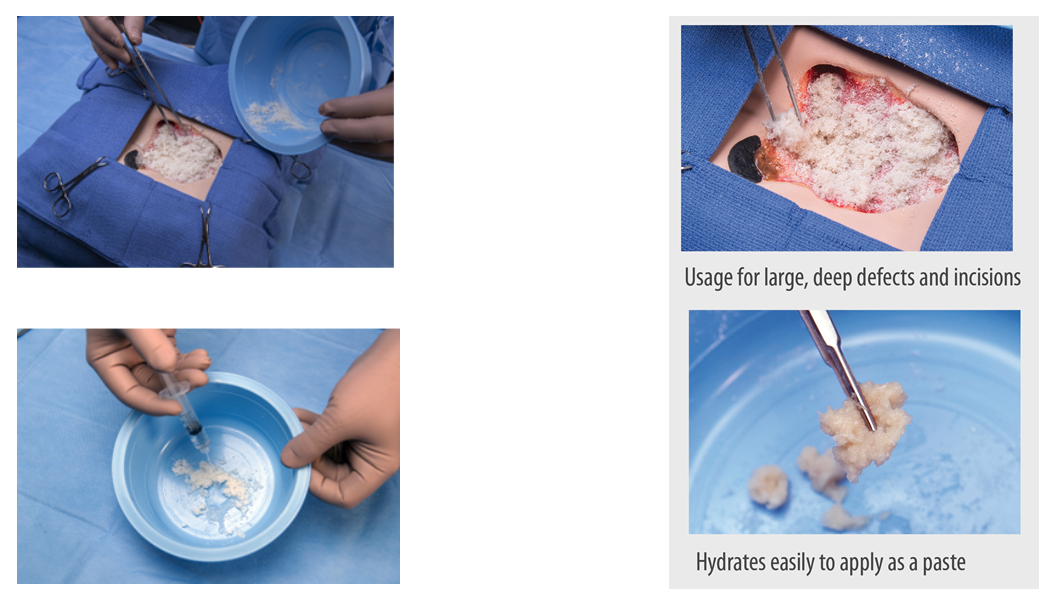
As a particulate, AXIOFILL presents a versatile option for various clinical applications and is easy to apply dry or hydrated to use in paste form.
A Replacement/Supplement of Damaged/Inadequate Integumental Tissue
AXIOFILL is intended for use in the replacement or supplementation of damaged or inadequate integumental tissue.
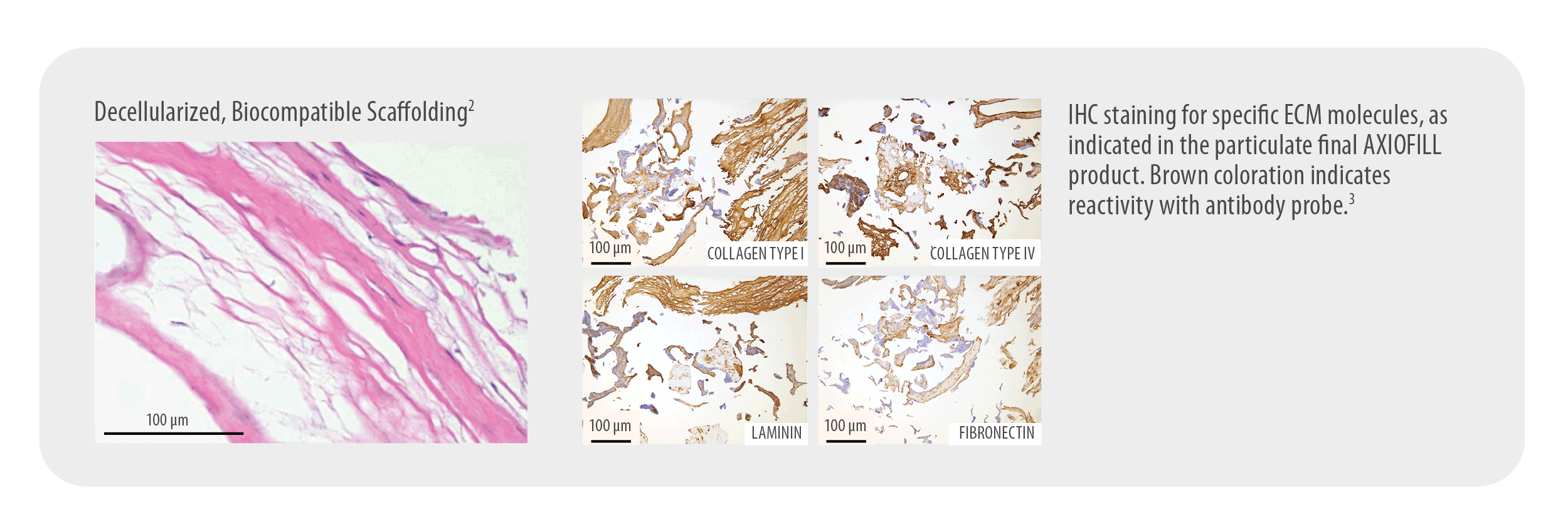
Provides a Biocompatible Extracellular Matrix
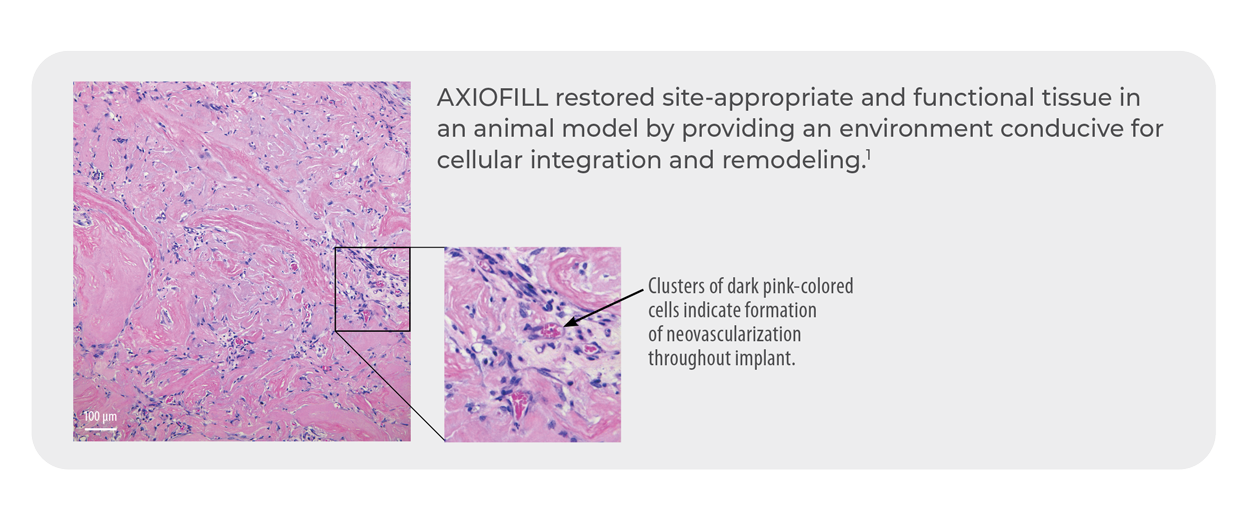
Gentle processing preserves the ECM components. Early scientific data in a nude mouse model shows AXIOFILL supports site appropriate function in tissue by allowing cell attachment, new collagen formation on the matrix, and new blood vessel formation.1
Product Advantages
- A lyophilized, biocompatible, and human extracellular matrix derived from the placental disc
- For use in the replacement or supplementation of damaged or inadequate integumental tissue
- Versatile and easy to use: apply dry or hydrated as a paste
- Economic alternative for larger soft tissue deficits
- Compatible with negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy (HBOT)
- Early scientific data in a nude mouse model shows AXIOFILL supports site appropriate function in tissue by allowing cell attachment, new collagen formation on the matrix, and new blood vessel formation1
Clinical Use Examples
AXIOFILL’s handling properties make it versatile for applying to complex, soft tissue deficits in its dry form, or as a paste. Clinical use examples may include:
Dry Applications
- Surgical debridement
- Partial and full thickness wounds
- Diabetic Foot Ulcers (DFUs)
- Venous Leg Ulcers (VLUs)
- Pressure Ulcers (PUs)
- Chronic vascular ulcers
- Trauma wounds (abrasions, lacerations, burns, and skin tears)
- Draining wounds
Hydrated/ Paste Applications
- Tunnel/undermined wounds
- Fistulae
- Deep, irregular wounds
- Incisions
- Surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence)
Product Application Options
AXIOFILL Processing & Details
AXIOFILL is processed with a unique patented method for placental-based allografts that is in accordance with the American Association of Tissue Banks (AATB) standards. The product is derived from placental tissue donated by healthy, consenting mothers who have given live Caesarean section birth in the US.
For an additional level of safety, the product is terminally sterilized.
References
- MIMEDX Internal Report. MM-RD-00113, Non-GLP Evaluation of Placental Based Products for Cellular Response in a Mouse Subcutaneous Implant.
- MIMEDX Internal Report. MM-RD-00112, Placental Collagen Matrix Development Phase Report.
- MIMEDX Internal Report. MM-RD-00105, Placental Collagen Matrix Feasibility Report.