What is AMNIOEFFECT?
AMNIOEFFECT® is a thick allograft comprised of human amnion, intermediate layer, and chorion. It is available in a wide variety of sizes to meet surgeons’ clinical needs.
AMNIOEFFECT Product Benefits
Protective Environment
AMNIOEFFECT provides a protective barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue in acute and chronic closures.
Regulatory Proteins
AMNIOEFFECT provides a biocompatible human extracellular matrix and contains 300+ regulatory proteins.1
Product Advantages
- Human-derived
- Thick graft allows for suturing to keep the graft in place and for placement in deeper surgical sites*
- Supports challenging closures in comorbid patients
- Simple storage and application
- Available in sheet configurations up to 180 cm2 to address a variety of surgical applications
- Compatible with negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy (HBOT)
Clinical Use Examples
AMNIOEFFECT has been used across a wide range of surgical applications including:
- Amputations
- Complex incision management
- Dehiscence repair
- Tendon & ligament repair
- Exposed bone or hardware
- Flaps
- Laminectomies
- Minimally Invasive Surgery (MIS)
- Hysterectomy
- Endometriosis
- Pilonidal cysts
*Not intended for use as a load bearing tissue.
Making a Difference
AMNIOEFFECT Processing & Details
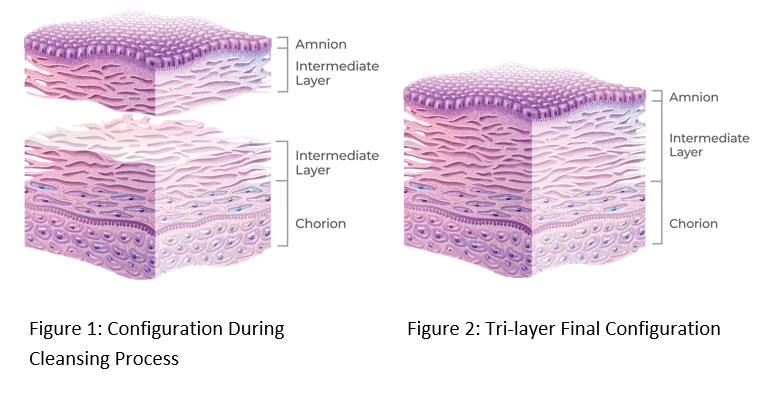
The PURION process has been optimized for retention of the Intermediate Layer (IL). Separation of the amnion and chorion, with attached IL (Figure 1), ensures thorough cleansing of each layer prior to lamination into the tri-layer final configuration (Figure 2).
Resources
Application Guide
MIMEDX Internal Report. MM-RD-00101, Development of Lyophilized Human Amnion Chorion Membrane.
Product Brochure
MIMEDX Advantage
We improve people’s health and lives through innovation that delivers solutions for patients and caregivers. With a wide variety of allograft configurations, clinicians can choose the product that best meets their treatment objectives.
Healthcare Professionals
MIMEDX offers unique value to clinicians across sites of care. With a broad support system, we offer services to support needs relating to reimbursement and coverage, product access and cost containment, education, patient information, and clinical applications.
References
- MIMEDX Internal Report. MM-RD-00101, Development of Lyophilized Human Amnion Chorion Membrane.