PEER-REVIEWED STUDY
Cranioplasty in AMNIOFIX Recipients
DOWNLOAD CLINICAL SUMMARY
Neurosurgeons report that dissections during cranioplasties for skull cap replacement can be time intensive and include risks to the patient when following conventional methods.1
Peer-reviewed RCTs and other studies have reported favorable results in patients undergoing dural repair in cranial and spine surgeries with human amniotic membrane allografts.2,3
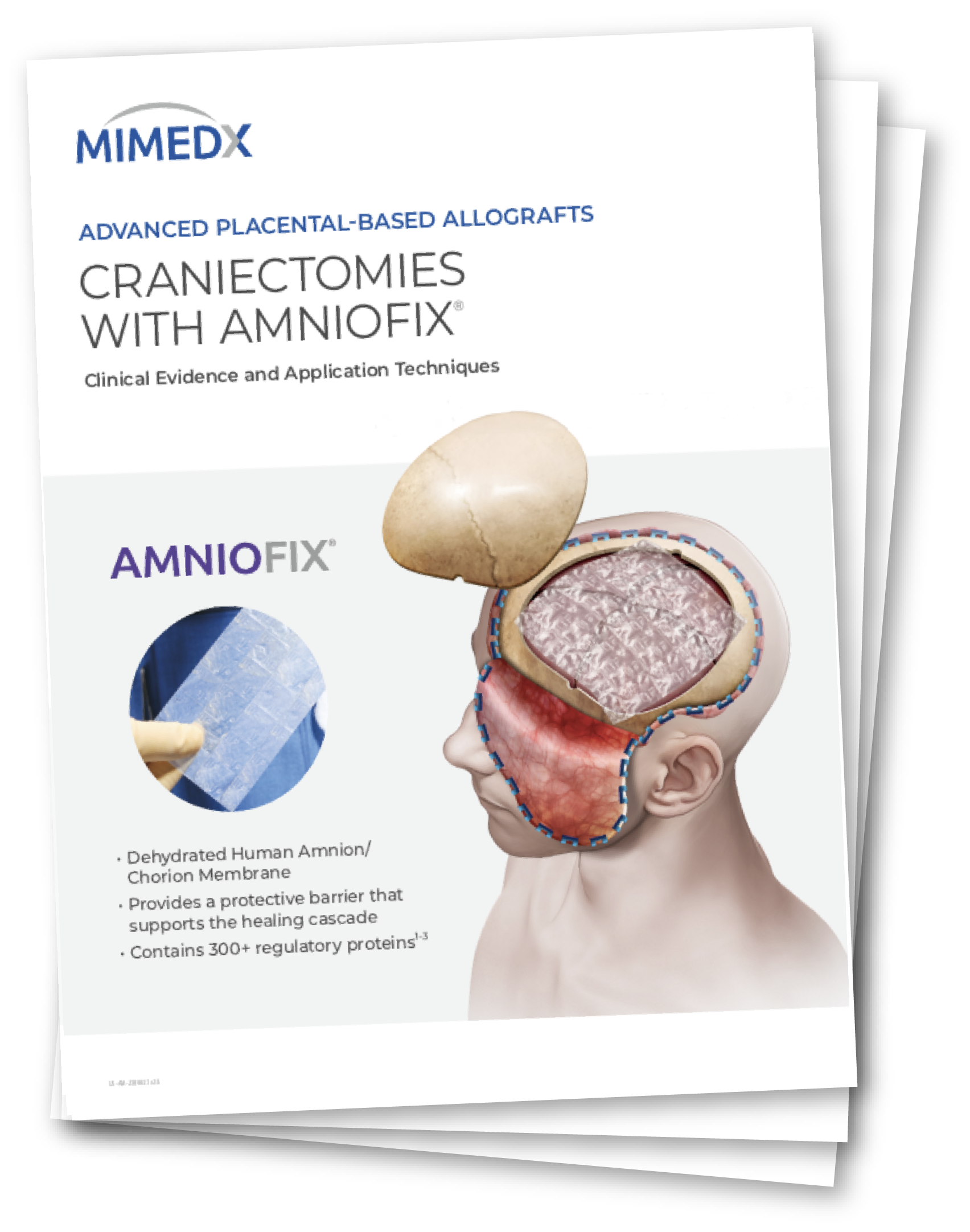
In a recent peer-reviewed study, AMNIOFIX was used as a protective barrier to support the healing cascade during emergent craniectomies.4
Key results during subsequent cranioplasty in AMNIOFIX recipients:4
- Under 3 minutes dissection time for skull flap replacement
- Minimal dural fibrosis in 86% of patients
- No operative complications


AMNIOFIX is a human amnion / chorion membrane that provides a protective barrier to support the healing cascade. The allograft contains 300+ regulatory proteins.5-7
References
- Griessenauer CJ, et al. World Neurosurg. 2015;84(6):2059-2063.
- Shah Z, et al. Surg Neurol Int. 2022;13:505. Published 2022 Nov 4.
- Subach BR, et al. Adv Orthop. 2015;2015:501202.
- Endicott L, et al. J Wound Care. 2023;32(10):634-640
- Koob TJ, et al. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
- Lei J, et al. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
- MIMEDX Internal Report. MM-RD-00086.
