Reimbursement

MIMEDX Reimbursement Support
Patient Insurance Verification
- Extensive Reimbursement Knowledge
- Experts in Coverage of MIMEDX Products
- Easy, User-friendly IVR Portal
- Fast Turnaround Time
Field Reimbursement Managers
- Coding & Billing Education
- Payer Coverage & Payer Mix Analysis
- Claims Review
- Appeals Support
Health Policy
- Over 300+ Million Coverage Lives
- 100% National Commercial Coverage for DFUs
MIMEDX Patient Insurance Verification
Helping patients is more than just our top priority; it informs everything we do. That means doing everything we can to accurately qualify insurance coverage. Our experienced Patient Insurance Verification Team can help you to both navigate the ins and outs of product coverage and eligibility and obtain prior authorization if required.
We offer flexible options to submit insurance verification requests, including secure faxing, the MIMEDX proprietary portal, or the Net Health Connections Module. If you would like to submit an insurance verification request, please sign up for MIMEDX CONNECT, download the form using the link below, or contact us for more information.
Reimbursement Programs Committed to the Success of Your Team
20+ Field Reimbursement Managers
20+ Insurance Verification Specialists
Flexible Options for IVR Submission
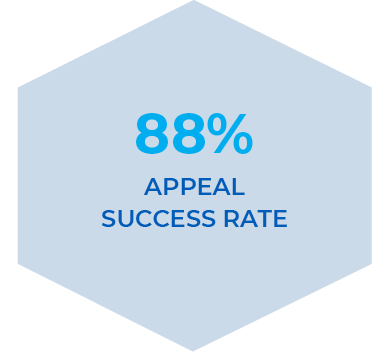
Appeals Support
Obtain Health Plan Eligibility, Benefits, and Authorizations
Available for On-Demand Education



A New Wound Care Management Portal
A first-of-its-kind online ordering and insurance verification tracking platform.
Contact Information
MIMEDX Patient Insurance Verification Team
855.882.8480
855.537.5825
BILLING & CODING
To receive a copy of our billing and coding guide, please contact your Field Reimbursement Manager or contact us at 855.882.8480.
HOPD Billing Guide
Physician Office Billing Guide
Patient Insurance Coverage
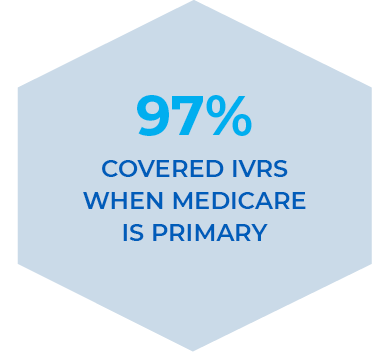
In addition to Medicare and Medicaid coverage, EPIFIX is now covered 100% among national plans for diabetic foot ulcers (DFUs).
Our product performance and supporting evidence has enabled best-in-class payer insurance coverage.
| MIMEDX PRODUCT COVERAGE | MIMEDX PRODUCT COVERAGE | OTHER AMNIOTIC PRODUCT COVERAGE | OTHER AMNIOTIC PRODUCT COVERAGE | |
| DIABETIC FOOT ULCER | VENOUS LEG ULCER | DIABETIC FOOT ULCER | VENOUS LEG ULCER | |
| UnitedHealthcare | Grafix | |||
| Aetna |
|
AmnioBand Grafix |
||
| Cigna | AmnioBand Grafix |
Grafix | ||
| Humana | Grafix | |||
| Anthem | AmnioBand Grafix |
|||
| BlueCross BlueShield |
AmnioBand
|
|||
| VA Community Care Network: Optum & Triwest FSS, MSPV, IDIQ & DAPA coverage |
|
|
Healthcare Professionals
MIMEDX offers unique value to clinicians across sites of care. With a broad support system, we offer services to support needs relating to reimbursement and coverage, product access and cost containment, education, patient information, and clinical applications.

